当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Temperature Affects the Selectivity of the Electrochemical CO2 Reduction on Copper
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acscatal.3c00706 Rafaël E Vos 1 , Kees E Kolmeijer 1 , Thimo S Jacobs 2 , Ward van der Stam 2 , Bert M Weckhuysen 2 , Marc T M Koper 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acscatal.3c00706 Rafaël E Vos 1 , Kees E Kolmeijer 1 , Thimo S Jacobs 2 , Ward van der Stam 2 , Bert M Weckhuysen 2 , Marc T M Koper 1
Affiliation
|
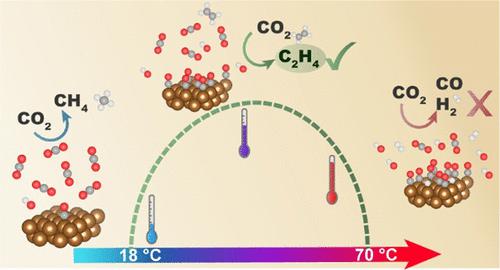
Copper is a unique catalyst for the electrochemical CO2 reduction reaction (CO2RR) as it can produce multi-carbon products, such as ethylene and propanol. As practical electrolyzers will likely operate at elevated temperatures, the effect of reaction temperature on the product distribution and activity of CO2RR on copper is important to elucidate. In this study, we have performed electrolysis experiments at different reaction temperatures and potentials. We show that there are two distinct temperature regimes. From 18 up to ∼48 °C, C2+ products are produced with higher Faradaic efficiency, while methane and formic acid selectivity decreases and hydrogen selectivity stays approximately constant. From 48 to 70 °C, it was found that HER dominates and the activity of CO2RR decreases. Moreover, the CO2RR products produced in this higher temperature range are mainly the C1 products, namely, CO and HCOOH. We argue that CO surface coverage, local pH, and kinetics play an important role in the lower-temperature regime, while the second regime appears most likely to be related to structural changes in the copper surface.
中文翻译:

温度如何影响铜上电化学 CO2 还原的选择性
铜是电化学CO 2的独特催化剂还原反应(CO2RR),因为它可以产生多碳产物,例如乙烯和丙醇。由于实际电解槽可能在高温下运行,因此阐明反应温度对铜上 CO2RR 的产物分布和活性的影响非常重要。在这项研究中,我们在不同的反应温度和电位下进行了电解实验。我们表明存在两种不同的温度状态。从 18 到 ∼48 °C,C2+ 产物的法拉第效率较高,而甲烷和甲酸选择性下降,而氢选择性基本保持恒定。从48℃到70℃,发现HER占主导地位,CO2RR活性下降。而且,在这个较高温度范围内产生的CO2RR产物主要是C1产物,即CO和HCOOH。
更新日期:2023-06-01
中文翻译:

温度如何影响铜上电化学 CO2 还原的选择性
铜是电化学CO 2的独特催化剂还原反应(CO2RR),因为它可以产生多碳产物,例如乙烯和丙醇。由于实际电解槽可能在高温下运行,因此阐明反应温度对铜上 CO2RR 的产物分布和活性的影响非常重要。在这项研究中,我们在不同的反应温度和电位下进行了电解实验。我们表明存在两种不同的温度状态。从 18 到 ∼48 °C,C2+ 产物的法拉第效率较高,而甲烷和甲酸选择性下降,而氢选择性基本保持恒定。从48℃到70℃,发现HER占主导地位,CO2RR活性下降。而且,在这个较高温度范围内产生的CO2RR产物主要是C1产物,即CO和HCOOH。











































 京公网安备 11010802027423号
京公网安备 11010802027423号