当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biophysical Properties of the Fibril Structure of the Toxic Conformer of Amyloid-β42: Characterization by Atomic Force Microscopy in Liquid and Molecular Docking
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acsami.3c06460 Radhika Biyani 1 , Kaito Hirata 2 , Kenji Oqmhula 3 , Ayhan Yurtsever 4 , Kenta Hongo 5 , Ryo Maezono 3 , Masahiro Takagi 1 , Takeshi Fukuma 4 , Manish Biyani 1, 6
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-06-01 , DOI: 10.1021/acsami.3c06460 Radhika Biyani 1 , Kaito Hirata 2 , Kenji Oqmhula 3 , Ayhan Yurtsever 4 , Kenta Hongo 5 , Ryo Maezono 3 , Masahiro Takagi 1 , Takeshi Fukuma 4 , Manish Biyani 1, 6
Affiliation
|
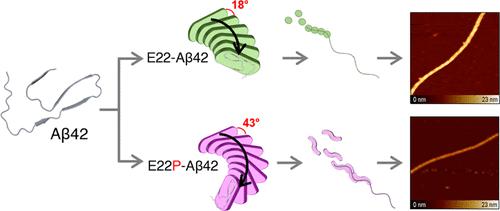
Alzheimer’s disease is associated with the aggregation of the misfolded neuronal peptide, amyloid-β42 (Aβ42). Evidence has suggested that several reasons are responsible for the toxicity caused by the aggregation of Aβ42, including the conformational restriction of Aβ42. In this study, one of the toxic conformers of Aβ42, which contains a Glu-to-Pro substitution (E22P-Aβ42), was explored using atomic force microscopy and molecular docking to study the aggregation dynamics. We proposed a systematic model of fibril formation to better understand the molecular basis of conformational transitions in the Aβ42 species. Our results demonstrated the formation of amorphous aggregates in E22P-Aβ42 that are stem-based, network-like structures, while the formation of mature fibrils occurred in the less toxic conformer of Aβ42, E22-Aβ42, that are sphere-like flexible structures. A comparison was made between the biophysical properties of E22P-Aβ42 and E22-Aβ42 that revealed that E22P-Aβ42 had greater stiffness, dihedral angle, number of β sheets involved, and elasticity, compared with E22-Aβ42. These findings will have considerable implications toward our understanding of the structural basis of the toxicity caused by conformational diversity in Aβ42 species.
中文翻译:

淀粉样蛋白-β42 毒性构象原纤维结构的生物物理特性:液体和分子对接中原子力显微镜的表征
阿尔茨海默病与错误折叠的神经元肽淀粉样蛋白-β42 (Aβ42) 的聚集有关。有证据表明,有几个原因导致 Aβ42 聚集引起的毒性,包括 Aβ42 的构象限制。在这项研究中,Aβ42 的一种有毒构象异构体包含 Glu-to-Pro 取代 (E22P-Aβ42),使用原子力显微镜和分子对接探索聚集动力学。我们提出了原纤维形成的系统模型,以更好地理解 Aβ42 物种构象转变的分子基础。我们的结果表明,E22P-Aβ42 中形成了基于茎的网络状结构的无定形聚集体,而成熟原纤维的形成发生在 Aβ42 的毒性较低的构象异构体 E22-Aβ42 中,是球状的柔性结构。对 E22P-Aβ42 和 E22-Aβ42 的生物物理特性进行比较表明,与 E22-Aβ42 相比,E22P-Aβ42 具有更大的刚度、二面角、涉及的 β 片层数和弹性。这些发现将对我们理解 Aβ42 物种的构象多样性引起的毒性的结构基础产生重大影响。
更新日期:2023-06-01
中文翻译:

淀粉样蛋白-β42 毒性构象原纤维结构的生物物理特性:液体和分子对接中原子力显微镜的表征
阿尔茨海默病与错误折叠的神经元肽淀粉样蛋白-β42 (Aβ42) 的聚集有关。有证据表明,有几个原因导致 Aβ42 聚集引起的毒性,包括 Aβ42 的构象限制。在这项研究中,Aβ42 的一种有毒构象异构体包含 Glu-to-Pro 取代 (E22P-Aβ42),使用原子力显微镜和分子对接探索聚集动力学。我们提出了原纤维形成的系统模型,以更好地理解 Aβ42 物种构象转变的分子基础。我们的结果表明,E22P-Aβ42 中形成了基于茎的网络状结构的无定形聚集体,而成熟原纤维的形成发生在 Aβ42 的毒性较低的构象异构体 E22-Aβ42 中,是球状的柔性结构。对 E22P-Aβ42 和 E22-Aβ42 的生物物理特性进行比较表明,与 E22-Aβ42 相比,E22P-Aβ42 具有更大的刚度、二面角、涉及的 β 片层数和弹性。这些发现将对我们理解 Aβ42 物种的构象多样性引起的毒性的结构基础产生重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号