当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, kinetic studies, and atom transfer reactivity of [2Fe–2E] model compounds
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2023-06-01 , DOI: 10.1039/d3qi00728f Erwin A. Weerawardhana 1 , Matthias Zeller 2 , Wei-Tsung Lee 1
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2023-06-01 , DOI: 10.1039/d3qi00728f Erwin A. Weerawardhana 1 , Matthias Zeller 2 , Wei-Tsung Lee 1
Affiliation
|
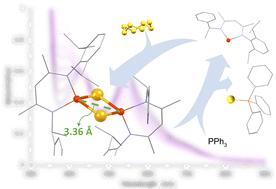
The synthesis of [2Fe–2E] (E = S and Se) complexes supported by an N-alkyl,N′-aryl-β-diketiminate ligand is described. The [2Fe–2S] model compound has an unusually long Fe⋯Fe distance, but the equivalent distance in the [2Fe–2Se] compound is comparable to those reported in the literature. These model compounds display varying electron transfer capabilities, which were elucidated through electronic spectroscopy and electrochemical analysis. Kinetic data and activation parameters suggest the formation mechanism of the [2Fe–2S] compound. Additionally, to probe the possible sulfur-transfer mechanism of [2Fe–2S] clusters, atom transfer reactivity was pursued.
中文翻译:

[2Fe–2E] 模型化合物的合成、动力学研究和原子转移反应性
描述了由N -烷基、N '-芳基-β-二酮亚胺配体支持的 [2Fe–2E](E = S 和 Se)配合物的合成。[2Fe–2S] 模型化合物具有异常长的 Fe⋯Fe 距离,但 [2Fe–2Se] 化合物中的等效距离与文献中报道的相当。这些模型化合物显示出不同的电子转移能力,这是通过电子光谱和电化学分析阐明的。动力学数据和活化参数表明 [2Fe–2S] 化合物的形成机制。此外,为了探究 [2Fe-2S] 团簇可能的硫转移机制,研究了原子转移反应性。
更新日期:2023-06-01
中文翻译:

[2Fe–2E] 模型化合物的合成、动力学研究和原子转移反应性
描述了由N -烷基、N '-芳基-β-二酮亚胺配体支持的 [2Fe–2E](E = S 和 Se)配合物的合成。[2Fe–2S] 模型化合物具有异常长的 Fe⋯Fe 距离,但 [2Fe–2Se] 化合物中的等效距离与文献中报道的相当。这些模型化合物显示出不同的电子转移能力,这是通过电子光谱和电化学分析阐明的。动力学数据和活化参数表明 [2Fe–2S] 化合物的形成机制。此外,为了探究 [2Fe-2S] 团簇可能的硫转移机制,研究了原子转移反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号