Journal of Hepatology ( IF 26.8 ) Pub Date : 2023-05-31 , DOI: 10.1016/j.jhep.2023.03.013 Banwari Agarwal 1 , Rafael Bañares Cañizares 2 , Faouzi Saliba 3 , Maria Pilar Ballester 4 , Dana Rodica Tomescu 5 , Daniel Martin 6 , Vanessa Stadlbauer 7 , Gavin Wright 8 , Mohammed Sheikh 9 , Carrie Morgan 10 , Carlos Alzola 11 , Phillip Lavin 12 , Daniel Green 10 , Rahul Kumar 13 , Sophie Caroline Sacleux 3 , Gernot Schilcher 7 , Sebastian Koball 14 , Andrada Tudor 15 , Jaak Minten 16 , Gema Domenech 17 , Juan Jose Aragones 17 , Karl Oettl 18 , Margret Paar 18 , Katja Waterstradt 19 , Stefanie M Bode-Boger 20 , Luis Ibáñez-Samaniego 21 , Amir Gander 22 , Carolina Ramos 23 , Alexandru Chivu 23 , Jan Stange 24 , Georg Lamprecht 25 , Moises Sanchez 26 , Rajeshwar P Mookerjee 9 , Andrew Davenport 9 , Nathan Davies 9 , Marco Pavesi 27 , Fausto Andreola 9 , Agustin Albillos 28 , Jeremy Cordingley 29 , Hartmut Schmidt 30 , Juan Antonio Carbonell-Asins 31 , Vicente Arroyo 27 , Javier Fernandez 32 , Steffen Mitzner 33 , Rajiv Jalan 34
|
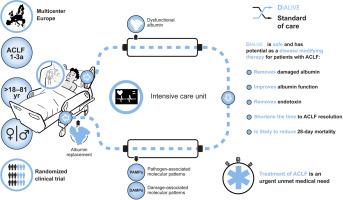
Background & Aims
Acute-on-chronic liver failure (ACLF) is characterized by severe systemic inflammation, multi-organ failure and high mortality rates. Its treatment is an urgent unmet need. DIALIVE is a novel liver dialysis device that aims to exchange dysfunctional albumin and remove damage- and pathogen-associated molecular patterns. This first-in-man randomized-controlled trial was performed with the primary aim of assessing the safety of DIALIVE in patients with ACLF, with secondary aims of evaluating its clinical effects, device performance and effect on pathophysiologically relevant biomarkers.
Methods
Thirty-two patients with alcohol-related ACLF were included. Patients were treated with DIALIVE for up to 5 days and end points were assessed at Day 10. Safety was assessed in all patients (n = 32). The secondary aims were assessed in a pre-specified subgroup that had at least three treatment sessions with DIALIVE (n = 30).
Results
There were no significant differences in 28-day mortality or occurrence of serious adverse events between the groups. Significant reduction in the severity of endotoxemia and improvement in albumin function was observed in the DIALIVE group, which translated into a significant reduction in the CLIF-C (Chronic Liver Failure consortium) organ failure (p = 0.018) and CLIF-C ACLF scores (p = 0.042) at Day 10. Time to resolution of ACLF was significantly faster in DIALIVE group (p = 0.036). Biomarkers of systemic inflammation such as IL-8 (p = 0.006), cell death [cytokeratin-18: M30 (p = 0.005) and M65 (p = 0.029)], endothelial function [asymmetric dimethylarginine (p = 0.002)] and, ligands for Toll-like receptor 4 (p = 0.030) and inflammasome (p = 0.002) improved significantly in the DIALIVE group.
Conclusions
These data indicate that DIALIVE appears to be safe and impacts positively on prognostic scores and pathophysiologically relevant biomarkers in patients with ACLF. Larger, adequately powered studies are warranted to further confirm its safety and efficacy.
Impact and implications
This is the first-in-man clinical trial which tested DIALIVE, a novel liver dialysis device for the treatment of cirrhosis and acute-on-chronic liver failure, a condition associated with severe inflammation, organ failures and a high risk of death. The study met the primary endpoint, confirming the safety of the DIALIVE system. Additionally, DIALIVE reduced inflammation and improved clinical parameters. However, it did not reduce mortality in this small study and further larger clinical trials are required to re-confirm its safety and to evaluate efficacy.
Clinical trial number
NCT03065699.
中文翻译:

DIALIVE 肝透析装置与慢性急性肝衰竭患者护理标准的随机对照临床试验
背景与目标
慢性肝衰竭(ACLF)的特点是严重的全身炎症、多器官衰竭和高死亡率。它的治疗是一个迫切的未满足的需求。 DIALIVE 是一种新型肝脏透析装置,旨在交换功能失调的白蛋白并消除损伤和病原体相关的分子模式。这项首次人体随机对照试验的主要目的是评估 DIALIVE 在 ACLF 患者中的安全性,次要目的是评估其临床效果、设备性能以及对病理生理学相关生物标志物的影响。
方法
32 名患有酒精相关 ACLF 的患者被纳入其中。患者接受 DIALIVE 治疗长达 5 天,并在第 10 天评估终点。对所有患者 (n = 32) 进行安全性评估。次要目标在预先指定的亚组中进行评估,该亚组至少接受过 3 次 DIALIVE 治疗(n = 30)。
结果
各组之间的 28 天死亡率或严重不良事件发生率没有显着差异。在 DIALIVE 组中观察到内毒素血症严重程度显着降低,白蛋白功能得到改善,这转化为 CLIF-C(慢性肝衰竭联盟)器官衰竭( p = 0.018)和 CLIF-C ACLF 评分显着降低(第 10 天( p = 0.042)。DIALIVE 组的 ACLF 消退时间明显更快( p = 0.036)。全身炎症的生物标志物,例如 IL-8 ( p = 0.006)、细胞死亡 [细胞角蛋白-18: M30 ( p = 0.005) 和 M65 ( p = 0.029)]、内皮功能 [不对称二甲基精氨酸 ( p = 0.002)] 以及, DIALIVE 组中 Toll 样受体 4 ( p = 0.030) 和炎症小体 ( p = 0.002) 的配体显着改善。
结论
这些数据表明 DIALIVE 似乎是安全的,并且对 ACLF 患者的预后评分和病理生理相关生物标志物产生积极影响。有必要进行更大规模、更有力的研究来进一步证实其安全性和有效性。
影响和影响
这是测试 DIALIVE 的首次人体临床试验,DIALIVE 是一种新型肝透析装置,用于治疗肝硬化和慢性肝衰竭(一种与严重炎症、器官衰竭和高死亡风险相关的疾病)。该研究达到了主要终点,证实了 DIALIVE 系统的安全性。此外,DIALIVE 还可以减少炎症并改善临床参数。然而,在这项小型研究中,它并没有降低死亡率,需要进一步更大规模的临床试验来重新确认其安全性并评估疗效。
临床试验编号
NCT03065699。









































 京公网安备 11010802027423号
京公网安备 11010802027423号