Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-06-01 , DOI: 10.1016/j.seppur.2023.124219 Binbin He , Yuanzhi Zhu , Yun Zu , Yunxiang Nie , Yi Mei
|
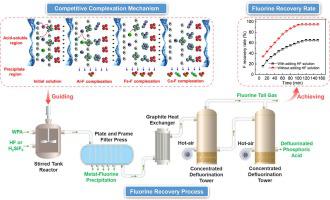
Fluorine (F) is a high-value resource that has been overlooked in the production of wet process phosphoric acid (WPA) due to its high susceptibility to complexation with metal cationic impurities, hindering F efficient recovery and subsequent phosphoric acid purification. This paper firstly emphasizes on investigating the competitive complexation behavior between F species and metal cations (M = Al3+, Fe3+, Ca2+ and Mg2+) by designing a series of F-containing model solutions (as HF and/or H2SiF6) with fixed combinations of metal cations. The results reveal that the complexation ability between metal cations and “free” F- species from HF dissociation and/or SiF62- hydrolysis follows the order: Al3+ > Fe3+ > Ca2+ > Mg2+. DFT calculation further confirms that the acid-soluble AlF4- and FeF3 species with lower binding energies are the main obstacle to difficult recovery of F species in the WPA. Given the above facts, an efficient F recovery strategy is proposed by increasing initial F concentration (as HF and H2SiF6) in industrial WPA for preferentially removing metal impurities in the forms of M−F precipitates (e.g., AlF3 and CaF2), according to the variations in distributions of M−F species under different n(F)/n(M) ratios predicted through establishment of mathematical models. The ultimate F recovery rate is greater than 95% by stripping process when initial HF concentration added is about 7.49 mol/L. This novel strategy has been successfully applied in phosphoric acid purification process.
中文翻译:

通过揭示氟物种与金属阳离子之间的竞争性络合行为,设计用于湿法磷酸纯化的有效氟回收策略
氟 (F) 是一种高价值资源,由于其高度易与金属阳离子杂质络合,阻碍了 F 的有效回收和后续磷酸纯化,因此在湿法磷酸 (WPA) 生产中一直被忽视。本文首先着重通过设计一系列含氟模型溶液(如HF和/或 H 2 SiF 6 ) 与金属阳离子的固定组合。结果表明,金属阳离子与“游离”F -来自 HF 解离和/或 SiF 6 2-水解的物质遵循以下顺序:Al 3+ > Fe 3+ > Ca 2+ > Mg 2+。DFT计算进一步证实,具有较低结合能的酸溶性AlF 4 -和FeF 3物种是WPA中F物种难以回收的主要障碍。鉴于上述事实,提出了一种有效的 F 回收策略,通过增加工业 WPA 中的初始 F 浓度(如 HF 和 H 2 SiF 6)优先去除 M−F 沉淀物形式的金属杂质(例如,AlF 3和 CaF 2),根据建立数学模型预测不同n(F)/n(M)比值下M−F物种分布的变化。当加入的HF初始浓度约为7.49 mol/L时,通过汽提工艺最终的F回收率大于95%。该新策略已成功应用于磷酸纯化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号