Water Research ( IF 11.4 ) Pub Date : 2023-05-31 , DOI: 10.1016/j.watres.2023.120156 Yishi Wang 1 , Wei Qiu 1 , Xiaohui Lu 2 , Xiaoqun Zhou 1 , Haochen Zhang 1 , Xiuxue Gong 3 , Baocai Gong 3 , Jun Ma 1
|
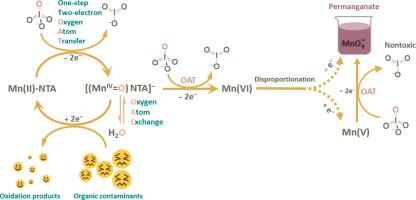
Periodate-based (PI, ) oxidation processes for pollutant elimination have gained increased attention in recent years. This study shows that nitrilotriacetic acid (NTA) can assist trace Mn(II) in activating PI for fast and long-lasting degradation of carbamazepine (CBZ) (100% degradation in 2 min). PI can oxidize Mn(II) to permanganate(, Mn(VII)) in the presence of NTA, which indicates the important role of transient manganese-oxo species. 18O isotope labeling experiments using methyl phenyl sulfoxide (PMSO) as a probe further confirmed the formation of manganese-oxo species. The chemical stoichiometric relationship (PI consumption: PMSO2 generation) and theoretical calculation suggested that Mn(IV)-oxo-NTA species were the main reactive species. The NTA-chelated manganese facilitated direct oxygen transfer from PI to Mn(II)-NTA and prevented hydrolysis and agglomeration of transient manganese-oxo species. PI was transformed completely to stable and nontoxic iodate but not lower-valent toxic iodine species (i.e., HOI, I2, and I−). The degradation pathways and mechanisms of CBZ were investigated using mass spectrometry and density functional theory (DFT) calculation. This study provided a steady and highly efficient choice for the quick degradation of organic micropollutants and broadened the perspective on the evolution mechanism of manganese intermediates in the Mn(II)/NTA/PI system.
中文翻译:

次氮基三乙酸辅助 Mn(II) 激活高碘酸盐快速和持久降解卡马西平:Mn(IV)-oxo 物种的重要性
基于高碘酸盐(PI,) 消除污染物的氧化过程近年来受到越来越多的关注。这项研究表明,次氮基三乙酸 (NTA) 可以协助痕量 Mn(II) 激活 PI,从而快速和持久地降解卡马西平 (CBZ)(2 分钟内 100% 降解)。PI可将Mn(II)氧化成高锰酸盐(, Mn(VII)) 在 NTA 存在下, 这表明瞬态锰氧物种的重要作用。使用甲基苯基亚砜 (PMSO) 作为探针的18 O 同位素标记实验进一步证实了锰氧物种的形成。化学计量关系(PI 消耗:PMSO 2代)和理论计算表明 Mn(IV)-oxo-NTA 物种是主要的活性物种。NTA 螯合的锰促进了从 PI 到 Mn(II)-NTA 的直接氧转移,并防止了瞬态锰氧物种的水解和团聚。PI 完全转化为稳定且无毒的碘酸盐,但未转化为低价有毒碘物质(即 HOI、I 2和 I -). 使用质谱和密度泛函理论 (DFT) 计算研究了 CBZ 的降解途径和机制。该研究为有机微污染物的快速降解提供了稳定高效的选择,拓宽了Mn(II)/NTA/PI体系中锰中间体演化机制的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号