Water Research ( IF 11.4 ) Pub Date : 2023-05-31 , DOI: 10.1016/j.watres.2023.120159 Ao Wang 1 , Shouliang Huo 2 , Jean-Philippe Croué 3 , Chao Liu 1
|
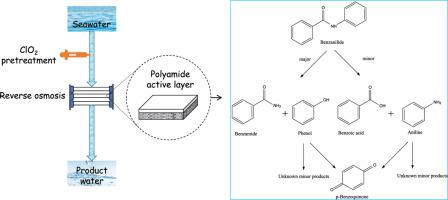
Aromatic polyamide (PA) based membranes are widely used for reverse osmosis (RO), but they can be degraded by free chlorine used for controlling the biofouling prior to RO treatment. Kinetics and mechanisms for the reactions of PA membrane model monomers, i.e., benzanilide (BA), and acetanilide (AC), with chlorine dioxide (ClO2) were investigated in this study. Rate constants for the reactions of ClO2 with BA and AC at pH 8.3 and 21°C were determined to be (4.1±0.1) × 10−1 M−1.24 s−1 and (6.0±0.1) × 10−3 M−1 s−1, respectively. These reactions are base assisted with a strong pH dependence. The activation energies of BA and AC degradation by ClO2 were 123.7 and 81.0 kJ mol−1, respectively. This indicates a relatively strong temperature dependence in the studied temperature range of 21–35 °C. The presence of bromide and natural organic matter does not promote the degradation of model monomers by ClO2. BA was degraded by ClO2 via two pathways: (1) the attack on the anilide moiety with the formation of benzamide (major pathway) and (2) oxidative hydrolysis to benzoic acid (minor pathway). A kinetic model was developed to simulate the degradation of BA and formation of byproducts during ClO2 pretreatment, and simulations agree well with the experimental data. Half-lives of BA treated by ClO2 were 1-5 orders of magnitude longer than chlorine under typical seawater treatment conditions. These novel findings suggest the potential application of ClO2 for controlling biofouling ahead of RO treatment at desalination treatments.
中文翻译:

聚酰胺膜模型单体与二氧化氯的反应:动力学、途径和意义
基于芳香族聚酰胺 (PA) 的膜广泛用于反渗透 (RO),但在反渗透处理之前,它们会被用于控制生物污垢的游离氯降解。本研究调查了PA 膜模型单体(即苯甲酰苯胺 (BA) 和乙酰苯胺 (AC))与二氧化氯 (ClO 2 ) 反应的动力学和机制。在 pH 8.3 和 21°C 下,ClO 2与 BA 和 AC的反应速率常数被确定为 (4.1±0.1) × 10 −1 M −1.24 s −1和 (6.0±0.1) × 10 −3 M − 1秒-1, 分别。这些反应是碱辅助的,具有很强的 pH 依赖性。BA 和 AC 被 ClO 2降解的活化能分别为 123.7 和 81.0 kJ mol -1。这表明在 21–35 °C 的研究温度范围内具有相对较强的温度依赖性。溴化物和天然有机物的存在不会促进模型单体被 ClO 2降解。BA 被 ClO 2通过两个途径降解:(1) 攻击苯胺部分并形成苯甲酰胺(主要途径)和 (2) 氧化水解为苯甲酸(次要途径)。开发了一个动力学模型来模拟在 ClO 2过程中 BA 的降解和副产物的形成预处理和模拟与实验数据吻合得很好。在典型的海水处理条件下,经过 ClO 2处理的 BA 的半衰期比氯长 1-5 个数量级。这些新发现表明,在海水淡化处理中,ClO 2可用于在反渗透处理之前控制生物污垢的潜在应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号