当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Computational Study on the Reaction Mechanism of Stereocontrolled Synthesis of β-Lactam within [2]Rotaxane
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.joc.3c00330 Rong Liang 1, 2 , Qinghai Zhou 1, 3 , Xin Li 1 , Ming Wah Wong 2 , Lung Wa Chung 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.joc.3c00330 Rong Liang 1, 2 , Qinghai Zhou 1, 3 , Xin Li 1 , Ming Wah Wong 2 , Lung Wa Chung 1
Affiliation
|
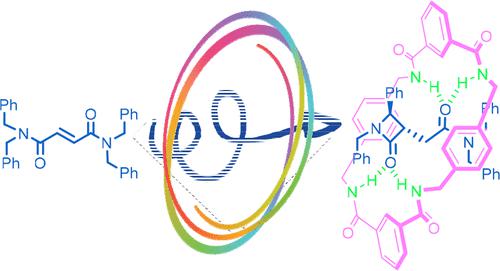
The macrocycle effect of [2]rotaxane on the highly trans-stereoselective cyclization reaction of N-benzylfumaramide was extensively investigated by various computational methods, including DFT and high-level DLPNO–CCSD(T) methods. Our computational results suggest that the most favorable mechanism of the CsOH-promoted cyclization of the fumaramide into trans-β-lactam within [2]rotaxane initiates with deprotonation of a N-benzyl group of the interlocked fumaramide substrate by CsOH, followed by the trans-selective C–C bond formation and protonation by one amide functional group of the macrocycle. Our distortion/interaction analysis further shows that the uncommon trans-stereoselective cyclization forming β-lactam within the rotaxane may be attributed to a higher distortion energy (mainly from the distortion of the twisted cis-fumaramide conformation enforced by the rotaxane). Our systematic study should give deeper mechanistic insight into the reaction mechanism influenced by a supramolecular host.
中文翻译:

[2]Rotaxane立体控制合成β-内酰胺反应机理的计算研究
通过各种计算方法,包括DFT和高水平DLPNO-CCSD(T)方法,广泛研究了[2]轮烷对N-苄基富马酰胺的高度反式立体选择性环化反应的大环效应。我们的计算结果表明,CsOH促进[2]轮烷内富马酰胺环化成反式-β-内酰胺的最有利机制始于CsOH对联锁富马酰胺底物的N-苄基的去质子化,然后是反式-通过大环的一个酰胺官能团选择性形成C-C键并质子化。我们的畸变/相互作用分析进一步表明,在轮烷内形成β-内酰胺的不常见的反式立体选择性环化可能归因于较高的畸变能(主要来自轮烷强制的扭曲顺式富马酰胺构象的畸变)。我们的系统研究应该对受超分子主体影响的反应机制有更深入的了解。
更新日期:2023-05-31
中文翻译:

[2]Rotaxane立体控制合成β-内酰胺反应机理的计算研究
通过各种计算方法,包括DFT和高水平DLPNO-CCSD(T)方法,广泛研究了[2]轮烷对N-苄基富马酰胺的高度反式立体选择性环化反应的大环效应。我们的计算结果表明,CsOH促进[2]轮烷内富马酰胺环化成反式-β-内酰胺的最有利机制始于CsOH对联锁富马酰胺底物的N-苄基的去质子化,然后是反式-通过大环的一个酰胺官能团选择性形成C-C键并质子化。我们的畸变/相互作用分析进一步表明,在轮烷内形成β-内酰胺的不常见的反式立体选择性环化可能归因于较高的畸变能(主要来自轮烷强制的扭曲顺式富马酰胺构象的畸变)。我们的系统研究应该对受超分子主体影响的反应机制有更深入的了解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号