当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Bonding of Halonium Compounds
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00654 Juan D Velasquez 1 , Jorge Echeverría 1 , Santiago Alvarez 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00654 Juan D Velasquez 1 , Jorge Echeverría 1 , Santiago Alvarez 2
Affiliation
|
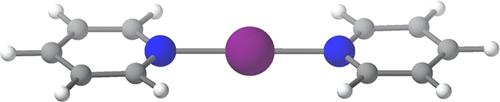
The geometrical parameters and the bonding in [D···X···D]+ halonium compounds, where D is a Lewis base with N as the donor atom and X is Cl, Br, or I, have been investigated through a combined structural and computational study. Cambridge Structural Database (CSD) searches have revealed linear and symmetrical [D···X···D]+ frameworks with neutral donors. By means of density functional theory (DFT), molecular electrostatic potential (MEP), and energy decomposition analyses (EDA) calculations, we have studied the effect of various halogen atoms (X) on the [D···X···D]+ framework, the effect of different nitrogen-donor groups (D) attached to an iodonium cation (X = I), and the influence of the electron density alteration on the [D···I···D]+ halonium bond by variation of the R substituents at the N-donor upon the symmetry, strength, and nature of the interaction. The physical origin of the interaction arises from a subtle interplay between electrostatic and orbital contributions (σ-hole bond). Interaction energies as high as 45 kcal/mol suggest that halonium bonds can be exploited for the development of novel halonium transfer agents, in asymmetric halofunctionalization or as building blocks in supramolecular chemistry.
中文翻译:

卤化合物的结构和成键
[D···X···D] +卤鎓化合物的几何参数和键合,其中 D 是路易斯碱,N 是供体原子,X 是 Cl、Br 或 I,通过联合研究结构和计算研究。剑桥结构数据库 (CSD) 搜索揭示了具有中性供体的线性和对称 [D···X···D] +框架。通过密度泛函理论(DFT)、分子静电势(MEP)和能量分解分析(EDA)计算,我们研究了各种卤素原子(X)对[D···X···D的影响] +框架,不同氮供体基团(D)与碘阳离子(X = I)的影响,以及电子密度变化对[D···I···D] + 的影响根据相互作用的对称性、强度和性质,N 供体上的 R 取代基发生变化而形成卤鎓键。相互作用的物理起源源于静电和轨道贡献(σ-空穴键)之间的微妙相互作用。高达 45 kcal/mol 的相互作用能表明,卤素键可用于开发新型卤素转移剂、不对称卤代功能化或作为超分子化学的构建单元。
更新日期:2023-05-31
中文翻译:

卤化合物的结构和成键
[D···X···D] +卤鎓化合物的几何参数和键合,其中 D 是路易斯碱,N 是供体原子,X 是 Cl、Br 或 I,通过联合研究结构和计算研究。剑桥结构数据库 (CSD) 搜索揭示了具有中性供体的线性和对称 [D···X···D] +框架。通过密度泛函理论(DFT)、分子静电势(MEP)和能量分解分析(EDA)计算,我们研究了各种卤素原子(X)对[D···X···D的影响] +框架,不同氮供体基团(D)与碘阳离子(X = I)的影响,以及电子密度变化对[D···I···D] + 的影响根据相互作用的对称性、强度和性质,N 供体上的 R 取代基发生变化而形成卤鎓键。相互作用的物理起源源于静电和轨道贡献(σ-空穴键)之间的微妙相互作用。高达 45 kcal/mol 的相互作用能表明,卤素键可用于开发新型卤素转移剂、不对称卤代功能化或作为超分子化学的构建单元。










































 京公网安备 11010802027423号
京公网安备 11010802027423号