当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of Carbon Dioxide to Formate by Noble Metal Catalysts Supported on a Chemically Stable Lanthanum Rod-Metal–Organic Framework
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00884 Maureen Gumbo 1, 2 , Banothile C E Makhubela 2 , Francoise M Amombo Noa 3 , Lars Öhrström 3 , Bassem Al-Maythalony 4 , Gift Mehlana 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00884 Maureen Gumbo 1, 2 , Banothile C E Makhubela 2 , Francoise M Amombo Noa 3 , Lars Öhrström 3 , Bassem Al-Maythalony 4 , Gift Mehlana 1
Affiliation
|
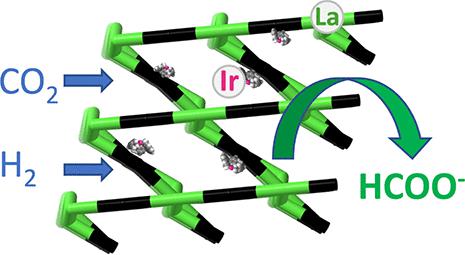
The conversion of carbon dioxide to formate is of great importance for hydrogen storage as well as being a step to access an array of olefins. Herein, we have prepared a JMS-5 metal–organic framework (MOF) using a bipyridyl dicarboxylate linker, with the molecular formula [La2(bpdc)3/2(dmf)2(OAc)3]·dmf. The MOF was functionalized by cyclometalation using Pd(II), Pt(II), Ru(II), Rh(III), and Ir(III) complexes. All metal catalysts supported on JMS-5 showed activity for CO2 hydrogenation to formate, with Rh(III)@JMS-5a and Ir(III)@JMS-5a yielding 4319 and 5473 TON, respectively. X-ray photoelectron spectroscopy of the most active catalyst Ir(III)@JMS-5a revealed that the iridium binding energies shifted to lower values, consistent with formation of Ir–H active species during catalysis. The transmission electron microscopy images of the recovered catalysts of Ir(III)@JMS-5a and Rh(III)@JMS-5a did not show any nanoparticles. This suggests that the catalytic activity observed was due to Ir(III) and Rh(III). The high activity displayed by Ir(III)@JMS-5a and Rh(III)@JMS-5a compared to using the Ir(III) and Rh(III) complexes on their own is attributed to the stabilization of the Ir(III) and Rh(III) on the nitrogen and carbon atom of the MOF backbone.
中文翻译:

化学稳定的镧棒-金属-有机骨架负载的贵金属催化剂将二氧化碳氢化为甲酸盐
二氧化碳转化为甲酸盐对于储氢非常重要,同时也是获取一系列烯烃的一个步骤。在此,我们使用二羧酸联吡啶连接基制备了 JMS-5 金属有机骨架 (MOF),分子式为 [La 2 ( bpdc ) 3/2 (dmf) 2 (OAc) 3 ]·dmf。使用 Pd(II)、Pt(II)、Ru(II)、Rh(III) 和 Ir(III) 络合物通过环金属化将 MOF 功能化。JMS-5 负载的所有金属催化剂均显示出对 CO 2的活性氢化成甲酸盐,Rh(III)@JMS-5a 和 Ir(III)@JMS-5a 分别产生 4319 和 5473 TON。活性最强的催化剂 Ir(III)@JMS-5a 的 X 射线光电子能谱表明,铱的结合能转移到较低的值,这与催化过程中 Ir-H 活性物质的形成一致。Ir(III)@JMS-5a 和 Rh(III)@JMS-5a 回收催化剂的透射电子显微镜图像未显示任何纳米颗粒。这表明观察到的催化活性是由于 Ir(III) 和 Rh(III)。与单独使用 Ir(III) 和 Rh(III) 配合物相比,Ir(III)@JMS-5a 和 Rh(III)@JMS-5a 表现出的高活性归因于 Ir(III) 的稳定性和 Rh(III) 在 MOF 主链的氮和碳原子上。
更新日期:2023-05-31
中文翻译:

化学稳定的镧棒-金属-有机骨架负载的贵金属催化剂将二氧化碳氢化为甲酸盐
二氧化碳转化为甲酸盐对于储氢非常重要,同时也是获取一系列烯烃的一个步骤。在此,我们使用二羧酸联吡啶连接基制备了 JMS-5 金属有机骨架 (MOF),分子式为 [La 2 ( bpdc ) 3/2 (dmf) 2 (OAc) 3 ]·dmf。使用 Pd(II)、Pt(II)、Ru(II)、Rh(III) 和 Ir(III) 络合物通过环金属化将 MOF 功能化。JMS-5 负载的所有金属催化剂均显示出对 CO 2的活性氢化成甲酸盐,Rh(III)@JMS-5a 和 Ir(III)@JMS-5a 分别产生 4319 和 5473 TON。活性最强的催化剂 Ir(III)@JMS-5a 的 X 射线光电子能谱表明,铱的结合能转移到较低的值,这与催化过程中 Ir-H 活性物质的形成一致。Ir(III)@JMS-5a 和 Rh(III)@JMS-5a 回收催化剂的透射电子显微镜图像未显示任何纳米颗粒。这表明观察到的催化活性是由于 Ir(III) 和 Rh(III)。与单独使用 Ir(III) 和 Rh(III) 配合物相比,Ir(III)@JMS-5a 和 Rh(III)@JMS-5a 表现出的高活性归因于 Ir(III) 的稳定性和 Rh(III) 在 MOF 主链的氮和碳原子上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号