Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2023-05-31 , DOI: 10.1016/j.jclepro.2023.137571 Xin He , Xin Yang , Chaoyang Zhang , Yuan Xiao , Yulin Tang
|
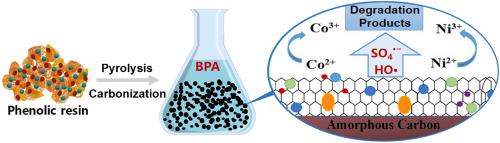
Bisphenol A (BPA) has caused adverse effects on human health and natural environment as a poisonous endocrine disrupting chemical. In this study, the bimetal composite carbon material (Co&Ni/C) with carbon nanotubes was fabricated for BPA degradation by the catalytic pyrolysis method. The dense carbon nanotubes were observed on the carbon surface with a specific surface area of up to 265.68 m2/g. The cobalt oxide and nickel oxide were distributed evenly causing more catalytic sites. The factors affecting catalytic efficiency for degrading BPA by the peroxymonosulfate (PMS) and hydrogen peroxide (H2O2) systems were investigated and compared systematically. The Co&Ni/C showed better catalytic performance with a BPA degradation rate of nearly 80%–100% in the Co&Ni/C-PMS process. However, BPA was difficult to be removed completely by H2O2 with a mineralization efficiency below 40% under most reaction conditions. The different degradation pathways and mechanisms under the oxidation of SO4•− and HO• were also discussed respectively, and the intermediate reactions included radical adduct formation, hydroxylation, skeleton oxidation rearrangement, dehydration and ring cleavage. It was proved that the stable Co&Ni/C possessed good reusability for remaining 77% removal rate of BPA after a 4-stage adsorption-degradation reaction. This paper revealed that the synthetic Co&Ni/C was hopeful as the adsorption material with efficient catalytic activity to provide new insight into BPA in-situ degradation.
中文翻译:

PMS 和 H2O2 体系中多相双金属复合碳催化降解双酚 A:性能和机理
双酚 A (BPA) 作为一种有毒的内分泌干扰化学物质,对人类健康和自然环境造成了不利影响。在这项研究中,制备了具有碳纳米管的双金属复合碳材料 (Co&Ni/C),用于通过催化热解法降解 BPA。在碳表面观察到致密的碳纳米管,比表面积高达265.68 m 2 /g。氧化钴和氧化镍分布均匀,导致更多的催化位点。影响过氧单硫酸盐(PMS)和过氧化氢(H 2 O 2 )降解BPA催化效率的因素) 系统进行了系统的调查和比较。Co&Ni/C 表现出更好的催化性能,在 Co&Ni/C-PMS 过程中 BPA 降解率接近 80%–100%。然而,在大多数反应条件下,BPA很难被H 2 O 2完全去除,矿化效率低于40%。SO 4氧化下的不同降解途径和机制• -和HO•也分别进行了讨论,中间反应包括自由基加合物形成、羟基化、骨架氧化重排、脱水和开环。事实证明,稳定的 Co&Ni/C 具有良好的可重复使用性,在经过 4 级吸附-降解反应后,BPA 的去除率仍为 77%。该论文表明,合成的 Co&Ni/C 有望作为具有高效催化活性的吸附材料,为 BPA 的原位降解提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号