当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diverse Site-Selective Transformation of Benzylic and Allylic Silyl Ethers via Organocatalytic Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acscatal.3c01153 Shohei Hamada 1 , Kaori Sakamoto 1 , Eri Miyazaki 1 , Elghareeb E. Elboray 1, 2 , Yusuke Kobayashi 1 , Takumi Furuta 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acscatal.3c01153 Shohei Hamada 1 , Kaori Sakamoto 1 , Eri Miyazaki 1 , Elghareeb E. Elboray 1, 2 , Yusuke Kobayashi 1 , Takumi Furuta 1
Affiliation
|
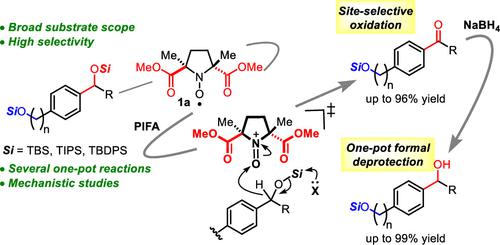
We herein report nitroxyl-radical catalyst 1a, which recognizes the electronic properties of silyl ethers, thus enabling selective oxidation of benzylic and allylic silyl ethers, despite steric factors. A subsequent one-pot reduction accomplishes the formal deprotection to the corresponding benzylic and allylic alcohols. This catalytic system allows the direct oxidative desymmetrization of bis-benzylic and bis-allylic silyl ethers to access synthetically useful monoprotected conjugated aldehydes, which can be applied to one-pot enantioselective transformations. Mechanistic studies revealed that the highly electron-accepting oxoammonium species from 1a as well as the trifluoroacetate form of phenyl iodonium bis(trifluoroacetate) (PIFA) play key roles for the selectivity-determining hydride transfer step.
中文翻译:

通过有机催化氧化对苯甲基和烯丙基硅醚进行多种位点选择性转化
我们在此报告了硝酰自由基催化剂1a,它识别甲硅烷基醚的电子特性,从而能够选择性氧化苄基和烯丙基甲硅烷基醚,尽管存在空间因素。随后的一锅还原完成了相应苯甲醇和烯丙醇的正式脱保护。该催化系统允许双苄基和双烯丙基甲硅烷基醚直接氧化去对称,以获得合成上有用的单保护共轭醛,其可应用于一锅对映选择性转化。机理研究表明, 1a中的高电子接受性氧铵物质以及三氟乙酸盐形式的苯基碘鎓双(三氟乙酸盐) (PIFA) 在决定选择性的氢化物转移步骤中发挥着关键作用。
更新日期:2023-05-31
中文翻译:

通过有机催化氧化对苯甲基和烯丙基硅醚进行多种位点选择性转化
我们在此报告了硝酰自由基催化剂1a,它识别甲硅烷基醚的电子特性,从而能够选择性氧化苄基和烯丙基甲硅烷基醚,尽管存在空间因素。随后的一锅还原完成了相应苯甲醇和烯丙醇的正式脱保护。该催化系统允许双苄基和双烯丙基甲硅烷基醚直接氧化去对称,以获得合成上有用的单保护共轭醛,其可应用于一锅对映选择性转化。机理研究表明, 1a中的高电子接受性氧铵物质以及三氟乙酸盐形式的苯基碘鎓双(三氟乙酸盐) (PIFA) 在决定选择性的氢化物转移步骤中发挥着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号