当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring Opening of Aziridines by Pendant Silanols Allows for Stereospecific Preparations of 1′-Amino-tetrahydrofurans
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-30 , DOI: 10.1021/acs.joc.3c00763 Appasaheb K Nirpal 1 , Someshwar Nagamalla 1 , Joel T Mague 2 , Shyam Sathyamoorthi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-30 , DOI: 10.1021/acs.joc.3c00763 Appasaheb K Nirpal 1 , Someshwar Nagamalla 1 , Joel T Mague 2 , Shyam Sathyamoorthi 1
Affiliation
|
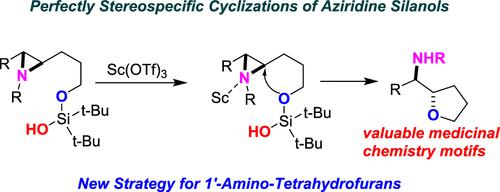
We have developed a highly stereospecific cyclization of aziridine silanols into 1′-amino-tetrahydrofurans. Our protocol of stirring a substrate with 10 mol % Sc (OTf)3 and 1 equivalent of NaHCO3 in CH2Cl2 is mild and compatible with a range of activating aziridine N-substituents (including tosylates, mesylates, and carbamates) and functional groups on the alkyl chains (including substituted aryl rings, alkyl bromides, and alkyl ethers). In all cases examined, trans di-substituted aziridine silanols give products with an erythro configuration; conversely, cis di-substituted aziridine silanols give products with a threo configuration. While literature syntheses of 1′-amino-tetrahydrofurans exist, only one example, contemporaneous with our work, uses a similar cyclization for their construction. Control experiments demonstrate that, for this transformation, the silanol is not particularly privileged, and a variety of protecting groups on the alcohol (including other silicon protecting groups, benzyl ethers, and MOM ethers) are compatible with product formation.
中文翻译:

通过侧链硅烷醇使氮丙啶开环,可以立体定向制备 1′-氨基四氢呋喃
我们开发了氮丙啶硅烷醇高度立体特异性环化成 1'-氨基四氢呋喃的方法。我们在 CH 2 Cl 2中搅拌底物与 10 mol% Sc (OTf) 3和 1 当量 NaHCO 3的方案是温和的,并且与一系列活化氮丙啶N取代基(包括甲苯磺酸盐、甲磺酸盐和氨基甲酸盐)和功能性相容。烷基链上的基团(包括取代的芳环、烷基溴和烷基醚)。在所有检查的情况下,反式二取代氮丙啶硅烷醇得到具有赤式构型的产物;相反,顺式二取代氮丙啶硅烷醇得到具有苏式的产物配置。虽然存在 1'-氨基-四氢呋喃的文献合成,但只有一个与我们的工作同时期的例子使用类似的环化来构建它们。对照实验表明,对于这种转化,硅烷醇并不是特别有利,并且醇上的各种保护基团(包括其他硅保护基团、苄基醚和MOM醚)与产物的形成相容。
更新日期:2023-05-30
中文翻译:

通过侧链硅烷醇使氮丙啶开环,可以立体定向制备 1′-氨基四氢呋喃
我们开发了氮丙啶硅烷醇高度立体特异性环化成 1'-氨基四氢呋喃的方法。我们在 CH 2 Cl 2中搅拌底物与 10 mol% Sc (OTf) 3和 1 当量 NaHCO 3的方案是温和的,并且与一系列活化氮丙啶N取代基(包括甲苯磺酸盐、甲磺酸盐和氨基甲酸盐)和功能性相容。烷基链上的基团(包括取代的芳环、烷基溴和烷基醚)。在所有检查的情况下,反式二取代氮丙啶硅烷醇得到具有赤式构型的产物;相反,顺式二取代氮丙啶硅烷醇得到具有苏式的产物配置。虽然存在 1'-氨基-四氢呋喃的文献合成,但只有一个与我们的工作同时期的例子使用类似的环化来构建它们。对照实验表明,对于这种转化,硅烷醇并不是特别有利,并且醇上的各种保护基团(包括其他硅保护基团、苄基醚和MOM醚)与产物的形成相容。











































 京公网安备 11010802027423号
京公网安备 11010802027423号