Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-05-30 , DOI: 10.1016/j.jhazmat.2023.131742 Mengzhu Yu 1 , Fumitake Takahashi 2 , Yaji Huang 3 , Zihong Li 4 , Hao Xu 2 , Pu Yang 2 , Zhicheng Zhu 3 , Lu Dong 5 , Conghui Fan 3 , Kunio Yoshikawa 2
|
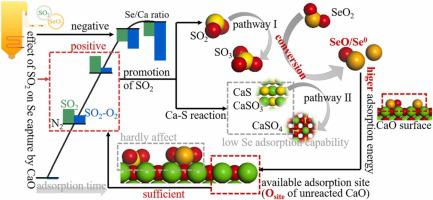
SO2 can noticeably impact the control of high toxic selenium emissions from flue gas by CaO. Surprisingly, our experiments showed that under certain conditions, SO2 can promote selenium capture by CaO, rather than hinder it. To elucidate the underlying mechanism, a combination of theoretical calculations and experiments was conducted. Thermodynamic equilibrium analysis revealed that gaseous SO2 and solid Ca-S reaction products can promote SeO2 converting to SeO/Se0. The Ca-S products facilitated greater SeO2 conversion compared to SO2. Experimental results demonstrated that selenium adsorption capacity of incompletely sulfurized CaO (CaO with pre-adsorbed SO2) was higher than that of completely sulfurized CaO (Ca-S products), highlighting the importance of adsorption sites of CaO. Density functional theory calculations showed that the pre-adsorbed SO2 hardly affected selenium adsorption energy on the SO2/CaO surface, while completely sulfurized CaO had low selenium adsorption energy, explaining the experimental phenomenon and proving necessary of CaO. Additionally, SeO/Se0 had higher adsorption energy on CaO than SeO2. Overall, the promotion of SO2 on selenium adsorption was primarily affected by two factors: 1) sulfur facilitating SeO2 conversion to SeO/Se0 which can be adsorbed more easily by CaO; 2) sufficient adsorption sites on CaO surface existing for SeO/Se0 adsorption, despite co-adsorption with sulfur.
中文翻译:

深入探讨CaO吸附过程中SO2对硒转化的作用:理论计算和实验研究
SO 2可以显着影响CaO对烟气中高毒硒排放的控制。令人惊讶的是,我们的实验表明,在一定条件下,SO 2可以促进而不是阻碍CaO对硒的捕获。为了阐明潜在的机制,进行了理论计算和实验的结合。热力学平衡分析表明,气态SO 2与固态Ca-S反应产物可以促进SeO 2转化为SeO/Se 0。与 SO 2相比, Ca-S 产品促进了更高的 SeO 2转化。实验结果表明,不完全硫化CaO(预吸附SO 2的CaO )对硒的吸附能力高于完全硫化CaO(Ca-S产品),凸显了CaO吸附位点的重要性。密度泛函理论计算表明,预吸附的SO 2对SO 2 /CaO表面的硒吸附能几乎没有影响,而完全硫化的CaO具有较低的硒吸附能,解释了实验现象并证明了CaO的必要性。此外,SeO/Se 0对CaO的吸附能比SeO 2更高。总体而言,SO 2的推广对硒吸附的影响主要受两个因素的影响:1)硫促进SeO 2转化为更容易被CaO吸附的SeO/Se 0 。2)尽管与硫共吸附,但CaO表面存在足够的吸附位点用于SeO/Se 0吸附。











































 京公网安备 11010802027423号
京公网安备 11010802027423号