Structure ( IF 4.4 ) Pub Date : 2023-05-29 , DOI: 10.1016/j.str.2023.05.003 Hyun Kyung Lee 1 , Yun-Tzai Lee 1 , Lixin Fan 2 , Haley M Wilt 1 , Chelsie E Conrad 1 , Ping Yu 1 , Jinwei Zhang 3 , Genbin Shi 4 , Xinhua Ji 4 , Yun-Xing Wang 1 , Jason R Stagno 1
|
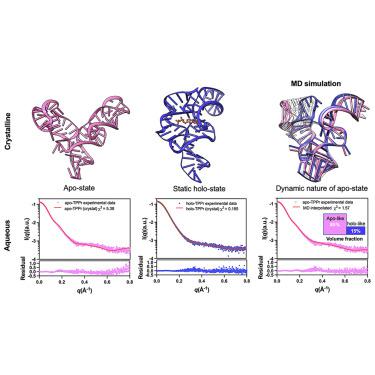
The thiamine pyrophosphate (TPP)-sensing riboswitch is one of the earliest discovered and most widespread riboswitches. Numerous structural studies have been reported for this riboswitch bound with various ligands. However, the ligand-free (apo) structure remains unknown. Here, we report a 3.1 Å resolution crystal structure of Escherichia coli TPP riboswitch in the apo state, which exhibits an extended, Y-shaped conformation further supported by small-angle X-ray scattering data and driven molecular dynamics simulations. The loss of ligand interactions results in helical uncoiling of P5 and disruption of the key tertiary interaction between the sensory domains. Opening of the aptamer propagates to the gene-regulatory P1 helix and generates the key conformational flexibility needed for the switching behavior. Much of the ligand-binding site at the three-way junction is unaltered, thereby maintaining a partially preformed pocket. Together, these results paint a dynamic picture of the ligand-induced conformational changes in TPP riboswitches that confer conditional gene regulation.
中文翻译:

apo 状态下大肠杆菌硫胺素焦磷酸感应核糖开关的晶体结构
焦磷酸硫胺素(TPP)传感核糖开关是最早发现和最广泛的核糖开关之一。对于这种与各种配体结合的核糖开关,已经报道了许多结构研究。然而,无配体(apo)结构仍然未知。在这里,我们报告了 apo 状态下大肠杆菌TPP 核糖开关的 3.1 Å 分辨率晶体结构,该结构表现出延伸的 Y 形构象,并得到小角度 X 射线散射数据和驱动分子动力学模拟的进一步支持。配体相互作用的丧失导致 P5 螺旋解旋并破坏感觉域之间的关键三级相互作用。适体的打开传播到基因调节 P1 螺旋,并产生转换行为所需的关键构象灵活性。三路连接处的许多配体结合位点未改变,从而保持了部分预先形成的口袋。总之,这些结果描绘了配体诱导的 TPP 核糖开关构象变化的动态图景,这些构象变化赋予条件基因调控。











































 京公网安备 11010802027423号
京公网安备 11010802027423号