当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The “Weak” C−H⋅⋅⋅S Interaction Drives Enantioselectivity in Cinchona Alkaloid Complex Catalyzed Thiocyanation
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2023-05-27 , DOI: 10.1002/asia.202300321 Subhrashis Banerjee 1, 2 , Kumar Vanka 1, 2
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2023-05-27 , DOI: 10.1002/asia.202300321 Subhrashis Banerjee 1, 2 , Kumar Vanka 1, 2
Affiliation
|
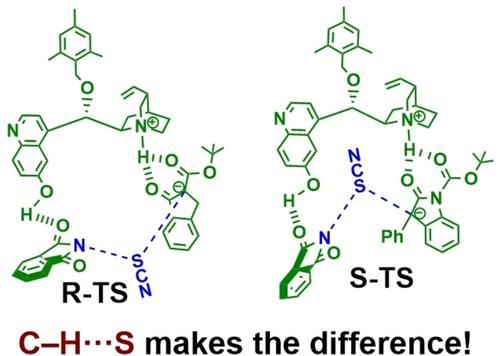
Computational studies with density functional theory (DFT) indicate that the supposedly “weak” C−H⋅⋅⋅S noncovalent interaction is the primary reason why enantioselectivity is reversed form R to S in the thiocyanation reaction with the asymmetric cinchona alkaloid complex (BzCPD) catalyst, when the nucleophile is changed from β-keto ester to oxindole. Since there are many asymmetric transformations that involve the sulphur heteroatom, the current findings have considerable relevance.
中文翻译:

“弱”C−H⋅⋅⋅S 相互作用驱动金鸡纳生物碱复合物催化硫氰化反应的对映选择性
密度泛函理论 (DFT) 的计算研究表明,所谓的“弱”C−H⋅⋅⋅S 非共价相互作用是在与不对称金鸡纳生物碱复合物 (BzCPD) 的硫氰化反应中对映选择性从 R 反转为 S 的主要原因当亲核试剂从β-酮酯转变为羟吲哚时,催化剂。由于存在许多涉及硫杂原子的不对称转变,因此当前的发现具有相当大的相关性。
更新日期:2023-05-27
中文翻译:

“弱”C−H⋅⋅⋅S 相互作用驱动金鸡纳生物碱复合物催化硫氰化反应的对映选择性
密度泛函理论 (DFT) 的计算研究表明,所谓的“弱”C−H⋅⋅⋅S 非共价相互作用是在与不对称金鸡纳生物碱复合物 (BzCPD) 的硫氰化反应中对映选择性从 R 反转为 S 的主要原因当亲核试剂从β-酮酯转变为羟吲哚时,催化剂。由于存在许多涉及硫杂原子的不对称转变,因此当前的发现具有相当大的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号