Industrial Crops and Products ( IF 5.6 ) Pub Date : 2023-05-27 , DOI: 10.1016/j.indcrop.2023.116905 Imran Khan Rind , Ahmet Sarı , Mustafa Tuzen , Muhammad Farooque Lanjwani , Tawfik A. Saleh
|
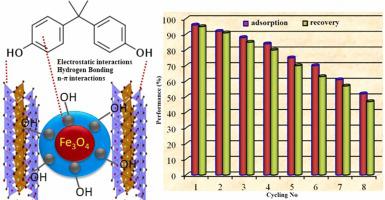
Bisphenol-A (BPA) is a conventional plasticizer and is considered a chemical of concern due to its potential for endocrine disruption. Therefore, bentonite (Bnt) clay-based silicon dioxide magnetic composite (Bnt/SiO2/Mag) was synthesized and used as an effective adsorbent for BPA removal from aqueous solutions. The factorial design study was applied for screening the experimental design of parameters. The isotherm modeling results indicated that the equilibrium data fitted to the non-linear Langmuir isotherm model with adoption capacity 276.5 mg g−1. The kinetic findings exhibited good suitability of pseudo-second-order (PSO) model with rate-limiting step involved in the chemical adsorption. The ΔG data between − 16.46 and − 18.02 kJ/mol for a temperature range of 293–323 K confirmed that the BPA adsorption process was taken place spontaneously. The ΔH parameter (−32.3 kJ/mol) characterized the exothermic nature of the adsorption process as the ΔS parameter (−53.44 J/mol.K) showed the reduced randomness at the solution-adsorbent interface after the adsorption process. The BPA adsorption can occur with a combination of different mechanisms including the filling of interlayer holes, electrostatic attraction, hydrogen bonds and π-π interaction. The cycling performance test demonstrated that Bnt/SiO2/Mag composite adsorbent still showed reasonable performance (70/75%) until five time-adsorption/desorption cycling. The results also designated that Bnt/SiO2/Mag composite can be valued as an alternative sorbent for the adsorption of BPA from water.
中文翻译:

膨润土/SiO2/磁铁矿纳米结构的合成作为从水中去除双酚 A 的高效吸附剂
双酚 A (BPA) 是一种传统的增塑剂,由于其可能破坏内分泌而被认为是一种令人担忧的化学品。因此,合成了膨润土 (Bnt) 粘土基二氧化硅磁性复合材料 (Bnt/SiO 2 /Mag),并将其用作从水溶液中去除 BPA 的有效吸附剂。因子设计研究用于筛选参数的实验设计。等温线建模结果表明,平衡数据符合采用容量为 276.5 mg g -1的非线性 Langmuir 等温线模型。动力学研究结果表明伪二级 (PSO) 模型具有良好的适用性,其中化学吸附涉及限速步骤。ΔG _在 293-323 K 的温度范围内 - 16.46 和 - 18.02 kJ/mol 之间的数据证实 BPA 吸附过程是自发发生的。ΔH参数 (-32.3 kJ/mol) 表征吸附过程的放热性质,因为 ΔS参数(-53.44 J/mol.K) 显示吸附过程后溶液-吸附剂界面处的随机性降低。BPA 吸附可以通过不同机制的组合发生,包括层间孔的填充、静电吸引、氢键和 π-π 相互作用。循环性能测试表明,Bnt/SiO 2 /Mag复合吸附剂在5次吸附/解吸循环后仍表现出合理的性能(70/75%)。结果还表明 Bnt/SiO2 /Mag 复合材料可用作从水中吸附 BPA 的替代吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号