当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Bonding Shuts Down Tunneling in Hydroxycarbenes: A Gas-Phase Study by Tandem-Mass Spectrometry, Infrared Ion Spectroscopy, and Theory
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-26 , DOI: 10.1021/jacs.3c01698 Mathias Paul 1 , Thomas Thomulka 1 , Wacharee Harnying 1 , Jörg-Martin Neudörfl 1 , Charlie R Adams 2 , Jonathan Martens 3 , Giel Berden 3 , Jos Oomens 3, 4 , Anthony J H M Meijer 2 , Albrecht Berkessel 1 , Mathias Schäfer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-26 , DOI: 10.1021/jacs.3c01698 Mathias Paul 1 , Thomas Thomulka 1 , Wacharee Harnying 1 , Jörg-Martin Neudörfl 1 , Charlie R Adams 2 , Jonathan Martens 3 , Giel Berden 3 , Jos Oomens 3, 4 , Anthony J H M Meijer 2 , Albrecht Berkessel 1 , Mathias Schäfer 1
Affiliation
|
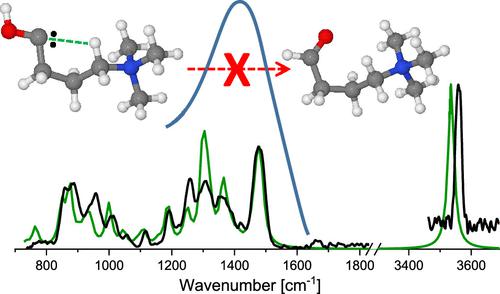
Hydroxycarbenes can be generated and structurally characterized in the gas phase by collision-induced decarboxylation of α-keto carboxylic acids, followed by infrared ion spectroscopy. Using this approach, we have shown earlier that quantum-mechanical hydrogen tunneling (QMHT) accounts for the isomerization of a charge-tagged phenylhydroxycarbene to the corresponding aldehyde in the gas phase and above room temperature. Herein, we report the results of our current study on aliphatic trialkylammonio-tagged systems. Quite unexpectedly, the flexible 3-(trimethylammonio)propylhydroxycarbene turned out to be stable─no H-shift to either aldehyde or enol occurred. As supported by density functional theory calculations, this novel QMHT inhibition is due to intramolecular H-bonding of a mildly acidic α-ammonio C–H bonds to the hydroxyl carbene’s C-atom (C:···H–C). To further support this hypothesis, (4-quinuclidinyl)hydroxycarbenes were synthesized, whose rigid structure prevents this intramolecular H-bonding. The latter hydroxycarbenes underwent “regular” QMHT to the aldehyde at rates comparable to, e.g., methylhydroxycarbene studied by Schreiner et al. While QMHT has been shown for a number of biological H-shift processes, its inhibition by H-bonding disclosed here may serve for the stabilization of highly reactive intermediates such as carbenes, even as a mechanism for biasing intrinsic selectivity patterns.
中文翻译:

氢键关闭羟基卡宾中的隧穿:通过串联质谱、红外离子光谱和理论进行的气相研究
羟基卡宾可以在气相中通过碰撞诱导的 α-酮基羧酸脱羧反应生成并进行结构表征,然后进行红外离子光谱分析。使用这种方法,我们之前已经表明,量子力学氢隧穿 (QMHT) 解释了电荷标记的苯基羟基卡宾在气相和室温以上异构化为相应的醛。在此,我们报告了我们目前对脂肪族三烷基氨标记系统的研究结果。出乎意料的是,柔性 3-(三甲基氨) 丙基羟基卡宾被证明是稳定的——没有 H 转移到醛或烯醇。在密度泛函理论计算的支持下,这种新型 QMHT 抑制作用是由于弱酸性 α-氨 C-H 键与羟基卡宾的 C 原子 (C: ···H-C)。为了进一步支持这一假设,合成了 (4-quinuclidinyl)hydroxycarbenes,其刚性结构阻止了这种分子内氢键。后一种羟基卡宾对醛进行“常规”QMHT,其速率与 Schreiner 等人研究的甲基羟基卡宾相当。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。Schreiner 等人研究的甲基羟基卡宾。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。Schreiner 等人研究的甲基羟基卡宾。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。
更新日期:2023-05-26
中文翻译:

氢键关闭羟基卡宾中的隧穿:通过串联质谱、红外离子光谱和理论进行的气相研究
羟基卡宾可以在气相中通过碰撞诱导的 α-酮基羧酸脱羧反应生成并进行结构表征,然后进行红外离子光谱分析。使用这种方法,我们之前已经表明,量子力学氢隧穿 (QMHT) 解释了电荷标记的苯基羟基卡宾在气相和室温以上异构化为相应的醛。在此,我们报告了我们目前对脂肪族三烷基氨标记系统的研究结果。出乎意料的是,柔性 3-(三甲基氨) 丙基羟基卡宾被证明是稳定的——没有 H 转移到醛或烯醇。在密度泛函理论计算的支持下,这种新型 QMHT 抑制作用是由于弱酸性 α-氨 C-H 键与羟基卡宾的 C 原子 (C: ···H-C)。为了进一步支持这一假设,合成了 (4-quinuclidinyl)hydroxycarbenes,其刚性结构阻止了这种分子内氢键。后一种羟基卡宾对醛进行“常规”QMHT,其速率与 Schreiner 等人研究的甲基羟基卡宾相当。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。Schreiner 等人研究的甲基羟基卡宾。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。Schreiner 等人研究的甲基羟基卡宾。虽然 QMHT 已被证明可用于许多生物 H 位移过程,但此处公开的其对氢键的抑制作用可用于稳定高反应性中间体(如卡宾),甚至作为偏置固有选择性模式的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号