当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brønsted Base Prompted sp3 C–H Latent Nucleophiles to Access α-Branched Amines Bearing β-Carbonyl by Cleaving Amide and Ester Bonds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.joc.3c00455 Ramdas Sreedharan 1 , Thirumanavelan Gandhi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.joc.3c00455 Ramdas Sreedharan 1 , Thirumanavelan Gandhi 1
Affiliation
|
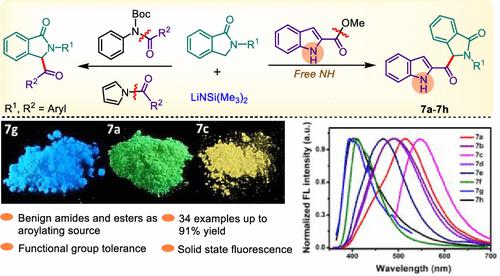
α-Branched amines are key motifs that exist in a plethora of natural products and pharmaceuticals. Herein we disclose the first convergent synthesis of α-branched amines bearing β-carbonyl in isoindolinones by employing unactivated tertiary amides and unactivated alkyl esters as benign electrophile sources. The reaction proceeds by the direct aroylation of a C(sp3)–H carbon adjacent to the nitrogen atom in core isoindolinones. Several amides and esters were screened to choose the potential acyl source for the substrate scope. The reaction is carried out with a repertoire of substrates under mild conditions and shows high functional group compatibility. Remarkably the reaction is amenable to organometallic ferrocenyl ester and indole methyl esters bearing an acidic NH moiety. Strikingly no trace of amidation product 8 is observed. In particular α-branched amines bearing β-carbonyl synthesized from indole methyl esters are considered important targets, as both motifs are prevalent in many drugs. This protocol is scalable, and products obtained from indole methyl esters show strong solid-state emission properties which are complementary with DFT calculations.
中文翻译:

布朗斯台德碱促使 sp3 C–H 潜在亲核试剂通过裂解酰胺键和酯键来接触带有 β-羰基的 α-支链胺
α-支化胺是存在于众多天然产物和药物中的关键基序。在此,我们公开了通过使用未活化的叔酰胺和未活化的烷基酯作为良性亲电试剂源,在异吲哚啉酮中首次聚合合成带有β-羰基的α-支化胺。该反应通过 C( sp 3)–与核心异吲哚啉酮中氮原子相邻的 H 碳。筛选了几种酰胺和酯,以选择底物范围的潜在酰基来源。该反应在温和条件下用一系列底物进行,并显示出高官能团相容性。值得注意的是,该反应适用于带有酸性 NH 部分的有机金属二茂铁酯和吲哚甲酯。令人惊讶的是没有观察到酰胺化产物8的痕迹。特别是由吲哚甲酯合成的带有 β-羰基的 α-支链胺被认为是重要的靶标,因为这两种基序在许多药物中都很常见。该协议具有可扩展性,从吲哚甲酯获得的产品显示出强大的固态发射特性,这与 DFT 计算互补。
更新日期:2023-05-25
中文翻译:

布朗斯台德碱促使 sp3 C–H 潜在亲核试剂通过裂解酰胺键和酯键来接触带有 β-羰基的 α-支链胺
α-支化胺是存在于众多天然产物和药物中的关键基序。在此,我们公开了通过使用未活化的叔酰胺和未活化的烷基酯作为良性亲电试剂源,在异吲哚啉酮中首次聚合合成带有β-羰基的α-支化胺。该反应通过 C( sp 3)–与核心异吲哚啉酮中氮原子相邻的 H 碳。筛选了几种酰胺和酯,以选择底物范围的潜在酰基来源。该反应在温和条件下用一系列底物进行,并显示出高官能团相容性。值得注意的是,该反应适用于带有酸性 NH 部分的有机金属二茂铁酯和吲哚甲酯。令人惊讶的是没有观察到酰胺化产物8的痕迹。特别是由吲哚甲酯合成的带有 β-羰基的 α-支链胺被认为是重要的靶标,因为这两种基序在许多药物中都很常见。该协议具有可扩展性,从吲哚甲酯获得的产品显示出强大的固态发射特性,这与 DFT 计算互补。











































 京公网安备 11010802027423号
京公网安备 11010802027423号