当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Bifunctional Ionic Liquid for Capture and Electrochemical Conversion of CO2 to CO over Silver
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01538 Saudagar Dongare 1 , Oguz Kagan Coskun 1 , Eda Cagli 1 , Kevin Y C Lee 2 , Guodong Rao 2 , R David Britt 2 , Louise A Berben 2 , Burcu Gurkan 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01538 Saudagar Dongare 1 , Oguz Kagan Coskun 1 , Eda Cagli 1 , Kevin Y C Lee 2 , Guodong Rao 2 , R David Britt 2 , Louise A Berben 2 , Burcu Gurkan 1
Affiliation
|
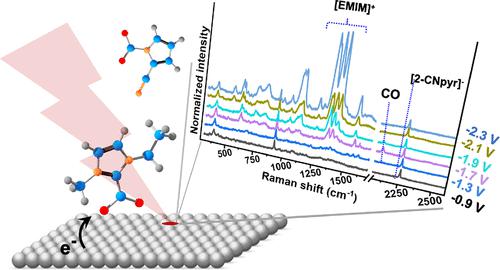
Electrochemical conversion of CO2 requires selective catalysts and high solubility of CO2 in the electrolyte to reduce the energy requirement and increase the current efficiency. In this study, the CO2 reduction reaction (CO2RR) over Ag electrodes in acetonitrile-based electrolytes containing 0.1 M [EMIM][2-CNpyr] (1-ethyl-3-methylimidazolium 2-cyanopyrolide), a reactive ionic liquid (IL), is shown to selectively (>94%) convert CO2 to CO with a stable current density (6 mA·cm–2) for at least 12 h. The linear sweep voltammetry experiments show the onset potential of CO2 reduction in acetonitrile shifts positively by 240 mV when [EMIM][2-CNpyr] is added. This is attributed to the pre-activation of CO2 through the carboxylate formation via the carbene intermediate of the [EMIM]+ cation and the carbamate formation via binding to the nucleophilic [2-CNpyr]− anion. The analysis of the electrode–electrolyte interface by surface-enhanced Raman spectroscopy (SERS) confirms the catalytic role of the functionalized IL where the accumulation of the IL-CO2 adduct between −1.7 and −2.3 V vs Ag/Ag+ and the simultaneous CO formation are captured. This study reveals the electrode surface species and the role of the functionalized ions in lowering the energy requirement of CO2RR for the design of multifunctional electrolytes for the integrated capture and conversion.
中文翻译:

用于在银上捕获 CO2 并将其电化学转化为 CO 的双功能离子液体
CO 2的电化学转化需要选择性催化剂和CO 2在电解质中的高溶解度,以减少能量需求并提高电流效率。在本研究中,在含有 0.1 M [EMIM][2-CNpyr](1-乙基-3-甲基咪唑鎓 2-氰基吡咯)(一种反应性离子液体)的乙腈基电解质中,在银电极上发生 CO 2还原反应 (CO 2 RR) (IL),显示可以在至少12小时内以稳定的电流密度(6 mA·cm –2 )选择性地(>94%)将CO 2转化为CO。线性扫描伏安实验表明,当添加[EMIM][2-CNpyr]时,乙腈中CO 2还原的起始电位正移240 mV。这归因于CO 2通过[EMIM] +阳离子的卡宾中间体形成羧酸盐以及通过与亲核[2-CNpyr] -阴离子结合形成氨基甲酸盐而预活化。通过表面增强拉曼光谱 (SERS) 对电极-电解质界面的分析证实了功能化 IL 的催化作用,其中 IL-CO 2加合物在 -1.7 和 -2.3 V vs Ag/Ag +之间积累,同时CO 的形成被捕获。这项研究揭示了电极表面物种和功能化离子在降低 CO 2 RR 能量需求方面的作用,以设计用于集成捕获和转化的多功能电解质。
更新日期:2023-05-25
中文翻译:

用于在银上捕获 CO2 并将其电化学转化为 CO 的双功能离子液体
CO 2的电化学转化需要选择性催化剂和CO 2在电解质中的高溶解度,以减少能量需求并提高电流效率。在本研究中,在含有 0.1 M [EMIM][2-CNpyr](1-乙基-3-甲基咪唑鎓 2-氰基吡咯)(一种反应性离子液体)的乙腈基电解质中,在银电极上发生 CO 2还原反应 (CO 2 RR) (IL),显示可以在至少12小时内以稳定的电流密度(6 mA·cm –2 )选择性地(>94%)将CO 2转化为CO。线性扫描伏安实验表明,当添加[EMIM][2-CNpyr]时,乙腈中CO 2还原的起始电位正移240 mV。这归因于CO 2通过[EMIM] +阳离子的卡宾中间体形成羧酸盐以及通过与亲核[2-CNpyr] -阴离子结合形成氨基甲酸盐而预活化。通过表面增强拉曼光谱 (SERS) 对电极-电解质界面的分析证实了功能化 IL 的催化作用,其中 IL-CO 2加合物在 -1.7 和 -2.3 V vs Ag/Ag +之间积累,同时CO 的形成被捕获。这项研究揭示了电极表面物种和功能化离子在降低 CO 2 RR 能量需求方面的作用,以设计用于集成捕获和转化的多功能电解质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号