当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed SN2 Glycosylation Employing an Amide-Functionalized 1-Naphthoate Platform Featuring a Selectivity-Safeguarding Mechanism
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c02792 Xu Ma 1 , Yongliang Zhang 1 , Xijun Zhu 1 , Yongliang Wei 1 , Liming Zhang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c02792 Xu Ma 1 , Yongliang Zhang 1 , Xijun Zhu 1 , Yongliang Wei 1 , Liming Zhang 1
Affiliation
|
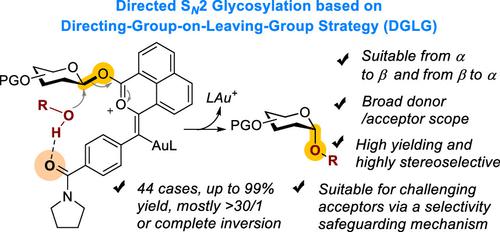
This work implements a catalytic SN2 glycosylation by employing an amide-functionalized 1-naphthoate platform as a latent glycosyl leaving group. Upon gold-catalyzed activation, the amide group enables the SN2 process by directing the attack of the glycosyl acceptor via H-bonding interaction, which results in stereoinversion at the anomeric center. Unique in this approach is that the amide group also enables a novel safeguarding mechanism by trapping oxocarbenium intermediates and, hence, minimizing stereorandom SN1 processes. The strategy is applicable to the synthesis of a broad range of glycosides with high to excellent levels of stereoinversion from anomerically pure/enriched glycosyl donors. These reactions are generally high-yielding, and their applications in the synthesis of challenging 1,2-cis-linkage-rich oligosaccharides are demonstrated.
中文翻译:

采用具有选择性保护机制的酰胺官能化 1-萘甲酸酯平台进行定向 SN2 糖基化
这项工作通过采用酰胺功能化的 1-萘甲酸酯平台作为潜在的糖基离去基团来实现催化 S N 2 糖基化。在金催化激活后,酰胺基团通过氢键相互作用引导糖基受体的攻击,从而实现 S N 2 过程,从而导致异头中心的立体倒转。这种方法的独特之处在于,酰胺基团还可以通过捕获氧碳鎓中间体来实现一种新颖的保护机制,从而最大限度地减少立体随机 S N 1 过程。该策略适用于从异头纯/富集糖基供体合成各种具有高至优异立体倒转水平的糖苷。这些反应通常是高产的,并且证明了它们在合成具有挑战性的富含 1,2-顺式键的寡糖中的应用。
更新日期:2023-05-25
中文翻译:

采用具有选择性保护机制的酰胺官能化 1-萘甲酸酯平台进行定向 SN2 糖基化
这项工作通过采用酰胺功能化的 1-萘甲酸酯平台作为潜在的糖基离去基团来实现催化 S N 2 糖基化。在金催化激活后,酰胺基团通过氢键相互作用引导糖基受体的攻击,从而实现 S N 2 过程,从而导致异头中心的立体倒转。这种方法的独特之处在于,酰胺基团还可以通过捕获氧碳鎓中间体来实现一种新颖的保护机制,从而最大限度地减少立体随机 S N 1 过程。该策略适用于从异头纯/富集糖基供体合成各种具有高至优异立体倒转水平的糖苷。这些反应通常是高产的,并且证明了它们在合成具有挑战性的富含 1,2-顺式键的寡糖中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号