当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkylcysteine Sulfoxide C–S Monooxygenase Uses a Flavin-Dependent Pummerer Rearrangement
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c03545 Sohan Hazra 1 , Tadhg P Begley 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-25 , DOI: 10.1021/jacs.3c03545 Sohan Hazra 1 , Tadhg P Begley 1
Affiliation
|
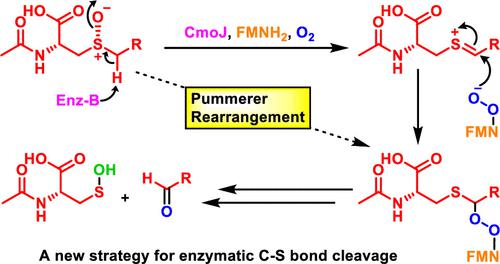
Flavoenzymes are highly versatile and participate in the catalysis of a wide range of reactions, including key reactions in the metabolism of sulfur-containing compounds. S-Alkyl cysteine is formed primarily by the degradation of S-alkyl glutathione generated during electrophile detoxification. A recently discovered S-alkyl cysteine salvage pathway uses two flavoenzymes (CmoO and CmoJ) to dealkylate this metabolite in soil bacteria. CmoO catalyzes a stereospecific sulfoxidation, and CmoJ catalyzes the cleavage of one of the sulfoxide C–S bonds in a new reaction of unknown mechanism. In this paper, we investigate the mechanism of CmoJ. We provide experimental evidence that eliminates carbanion and radical intermediates and conclude that the reaction proceeds via an unprecedented enzyme-mediated modified Pummerer rearrangement. The elucidation of the mechanism of CmoJ adds a new motif to the flavoenzymology of sulfur-containing natural products and demonstrates a new strategy for the enzyme-catalyzed cleavage of C–S bonds.
中文翻译:

烷基半胱氨酸亚砜 C-S 单加氧酶使用黄素依赖性 Pummerer 重排
黄素酶用途广泛,参与多种反应的催化,包括含硫化合物代谢中的关键反应。 S-烷基半胱氨酸主要由亲电子解毒过程中产生的S-烷基谷胱甘肽降解形成。最近发现的 S-烷基半胱氨酸补救途径使用两种黄素酶(CmoO 和 CmoJ)使土壤细菌中的这种代谢物脱烷基化。 CmoO 催化立体定向磺化氧化,CmoJ 在未知机制的新反应中催化其中一个亚砜 C-S 键的断裂。在本文中,我们研究了 CmoJ 的机制。我们提供了消除碳负离子和自由基中间体的实验证据,并得出结论,该反应是通过前所未有的酶介导的修饰普默勒重排进行的。 CmoJ机制的阐明为含硫天然产物的黄酮酶学添加了新的基序,并展示了酶催化C-S键断裂的新策略。
更新日期:2023-05-25
中文翻译:

烷基半胱氨酸亚砜 C-S 单加氧酶使用黄素依赖性 Pummerer 重排
黄素酶用途广泛,参与多种反应的催化,包括含硫化合物代谢中的关键反应。 S-烷基半胱氨酸主要由亲电子解毒过程中产生的S-烷基谷胱甘肽降解形成。最近发现的 S-烷基半胱氨酸补救途径使用两种黄素酶(CmoO 和 CmoJ)使土壤细菌中的这种代谢物脱烷基化。 CmoO 催化立体定向磺化氧化,CmoJ 在未知机制的新反应中催化其中一个亚砜 C-S 键的断裂。在本文中,我们研究了 CmoJ 的机制。我们提供了消除碳负离子和自由基中间体的实验证据,并得出结论,该反应是通过前所未有的酶介导的修饰普默勒重排进行的。 CmoJ机制的阐明为含硫天然产物的黄酮酶学添加了新的基序,并展示了酶催化C-S键断裂的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号