当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multienzyme-Mimicking LaCoO3 Nanotrigger for Programming Cancer-Cell Pyroptosis
Advanced Materials ( IF 27.4 ) Pub Date : 2023-05-25 , DOI: 10.1002/adma.202302961 Ke Xu 1 , Meiqi Chang 2 , Zeyu Wang 3 , Haitang Yang 1 , Yunxuan Jia 1 , Weijiao Xu 1 , Baicheng Zhao 1 , Yu Chen 3 , Feng Yao 1, 4
Advanced Materials ( IF 27.4 ) Pub Date : 2023-05-25 , DOI: 10.1002/adma.202302961 Ke Xu 1 , Meiqi Chang 2 , Zeyu Wang 3 , Haitang Yang 1 , Yunxuan Jia 1 , Weijiao Xu 1 , Baicheng Zhao 1 , Yu Chen 3 , Feng Yao 1, 4
Affiliation
|
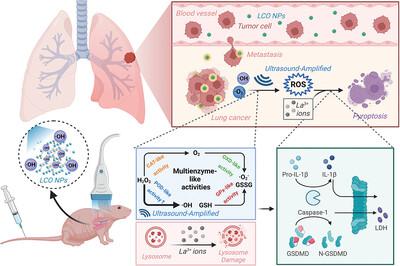
Pyroptosis, a distinct paradigm of programmed cell death, is an efficient strategy against cancer by overcoming resistance to apoptosis. In this study, LaCoO3 (LCO) lanthanide-based nanocrystals with multienzyme characteristics are rationally designed and engineered to trigger the generation of cytotoxic reactive oxygen species (ROS) and the release of lanthanum ions, ultimately inducing lung cancer cell pyroptosis. The peroxidase- and oxidase-mimicking activities of LCO nanocrystals endow LCO with ROS production capacity in tumor tissues with an acidic pH and high hydrogen peroxide content. Concurrently, the LCO nanoenzyme exhibits catalase- and glutathione peroxidase-like activities, reversing the hypoxic microenvironment, destroying the activated antioxidant system of tumor cells, and amplifying the sensitivity of tumor cells to ROS. The use of ultrasound further accelerates the enzymatic kinetic rate. Most importantly, the La3+ ions released by LCO robustly destroy the lysosomal membrane, finally inducing canonical pyroptotic cell death, together with ROS. LCO-nanocrystal-triggered programmed cell pyroptosis amplifies the therapeutic effects both in vitro and in vivo, effectively restraining lung cancer growth and metastasis. This study paves a new avenue for the efficient treatment of lung cancer and metastasis through US-enhanced lanthanum-based nanoenzyme platforms and pyroptotic cell death.
中文翻译:

多酶模拟 LaCoO3 纳米触发器用于编程癌细胞焦亡
焦亡是程序性细胞死亡的一种独特范例,是通过克服细胞凋亡抵抗来对抗癌症的有效策略。在这项研究中,具有多酶特性的LaCoO 3 (LCO)镧系纳米晶体被合理设计和改造,以触发细胞毒性活性氧(ROS)的产生和镧离子的释放,最终诱导肺癌细胞焦亡。LCO纳米晶体的过氧化物酶和氧化酶模拟活性赋予LCO在酸性pH和高过氧化氢含量的肿瘤组织中产生ROS的能力。同时,LCO纳米酶表现出类似过氧化氢酶和谷胱甘肽过氧化物酶的活性,逆转缺氧微环境,破坏肿瘤细胞激活的抗氧化系统,增强肿瘤细胞对ROS的敏感性。超声波的使用进一步加速了酶的动力学速率。最重要的是, LCO 释放的La 3+离子强烈破坏溶酶体膜,最终与 ROS 一起诱导典型的焦亡细胞死亡。LCO纳米晶触发的程序性细胞焦亡增强了体外和体内的治疗效果,有效抑制肺癌的生长和转移。这项研究通过美国增强的基于镧的纳米酶平台和焦亡细胞死亡,为有效治疗肺癌和转移铺平了一条新途径。
更新日期:2023-05-25
中文翻译:

多酶模拟 LaCoO3 纳米触发器用于编程癌细胞焦亡
焦亡是程序性细胞死亡的一种独特范例,是通过克服细胞凋亡抵抗来对抗癌症的有效策略。在这项研究中,具有多酶特性的LaCoO 3 (LCO)镧系纳米晶体被合理设计和改造,以触发细胞毒性活性氧(ROS)的产生和镧离子的释放,最终诱导肺癌细胞焦亡。LCO纳米晶体的过氧化物酶和氧化酶模拟活性赋予LCO在酸性pH和高过氧化氢含量的肿瘤组织中产生ROS的能力。同时,LCO纳米酶表现出类似过氧化氢酶和谷胱甘肽过氧化物酶的活性,逆转缺氧微环境,破坏肿瘤细胞激活的抗氧化系统,增强肿瘤细胞对ROS的敏感性。超声波的使用进一步加速了酶的动力学速率。最重要的是, LCO 释放的La 3+离子强烈破坏溶酶体膜,最终与 ROS 一起诱导典型的焦亡细胞死亡。LCO纳米晶触发的程序性细胞焦亡增强了体外和体内的治疗效果,有效抑制肺癌的生长和转移。这项研究通过美国增强的基于镧的纳米酶平台和焦亡细胞死亡,为有效治疗肺癌和转移铺平了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号