当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Unstrained Ketone C(O)–C Bond: 1,2-Nucleophilic Addition Followed by β-Carbon Elimination Strategy
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01602 Qiang Li 1 , Yiwen Xu 1 , Yang Long 2 , Sun Li 3 , Buyi Xu 4 , Ying Xia 5 , Xiangge Zhou 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01602 Qiang Li 1 , Yiwen Xu 1 , Yang Long 2 , Sun Li 3 , Buyi Xu 4 , Ying Xia 5 , Xiangge Zhou 1
Affiliation
|
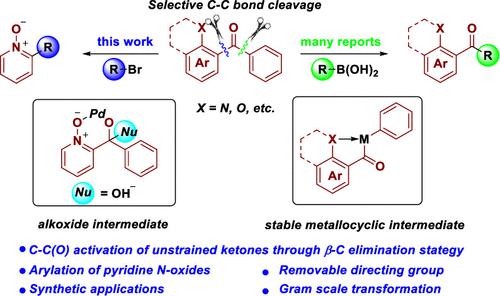
Transition-metal-catalyzed C–C bond activation has emerged as an increasingly effective method for reorganizing a molecular skeleton. Ketone, as a versatile functional molecule, has been extensively studied for the C–C bond activation reaction. However, most C–C bond activation of unstrained ketones generally follows an oxidative addition strategy, while a nucleophilic 1,2-addition of a carbonyl moiety followed by β-C elimination strategy was less studied. Here a palladium-catalyzed C–C bond activation of an unstrained ketone enabled by a removable directing group through β-C elimination to synthesize 2-arylpyridine is described. The protocol features wide substrate scope with yields up to 95%, good functional group tolerance, and functionality of natural products. The 2-arylpyridine N-oxide products can be easily converted to the corresponding 2-arylpyridines under mild reaction conditions.
中文翻译:

无张力酮 C(O)–C 键的活化:1,2-亲核加成,然后是 β-碳消除策略
过渡金属催化的 C-C 键活化已成为一种日益有效的分子骨架重组方法。酮作为一种多功能的功能分子,其 C-C 键活化反应已被广泛研究。然而,大多数无张力酮的C-C键活化通常遵循氧化加成策略,而羰基部分的亲核1,2-加成随后β-C消除策略的研究较少。这里描述了钯催化的无张力酮的 C-C 键活化,通过可去除的导向基团通过 β-C 消除来合成 2-芳基吡啶。该方案具有底物范围宽、收率高达95%、良好的官能团耐受性以及天然产物的功能等特点。2-芳基吡啶N-氧化物产物在温和的反应条件下可以很容易地转化为相应的2-芳基吡啶。
更新日期:2023-05-25
中文翻译:

无张力酮 C(O)–C 键的活化:1,2-亲核加成,然后是 β-碳消除策略
过渡金属催化的 C-C 键活化已成为一种日益有效的分子骨架重组方法。酮作为一种多功能的功能分子,其 C-C 键活化反应已被广泛研究。然而,大多数无张力酮的C-C键活化通常遵循氧化加成策略,而羰基部分的亲核1,2-加成随后β-C消除策略的研究较少。这里描述了钯催化的无张力酮的 C-C 键活化,通过可去除的导向基团通过 β-C 消除来合成 2-芳基吡啶。该方案具有底物范围宽、收率高达95%、良好的官能团耐受性以及天然产物的功能等特点。2-芳基吡啶N-氧化物产物在温和的反应条件下可以很容易地转化为相应的2-芳基吡啶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号