当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical Perfluoroalkylation of Aliphatic Substrates
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01922 Damian E. Yerien 1 , Sebastian Barata-Vallejo 1, 2 , Al Postigo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-25 , DOI: 10.1021/acscatal.3c01922 Damian E. Yerien 1 , Sebastian Barata-Vallejo 1, 2 , Al Postigo 1
Affiliation
|
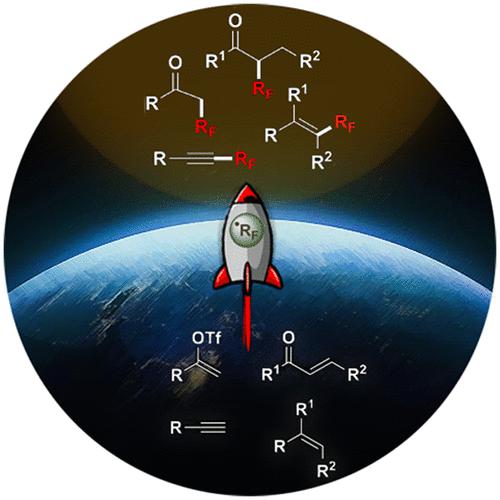
A selection of perfluoroalkylation reactions of aliphatic substrates that display methodological and synthetic amplitude will be studied, giving examples of their applications and mechanistic details. An array of synthetic approaches for fluoroalkylation reactions are documented; in particular, radical protocols are prominent among perfluoroalkylation methods. To that effect, addition reactions of perfluoroalkyl radicals RF (RF=CnF2n+1, n > 1) to unsaturated organic substrates serve as one of the most direct and efficacious ways to access fluoroalkylated scaffolds. The syntheses of perfluoroalkyl-substituted vinyl, alkynyl, and allylic compounds; the syntheses of perfluoroalkyl-substituted hydro-, iodo-, and oxy-perfluoroalkylated alkanes and olefins; the syntheses of perfluoroalkyl-substituted carbonyl compounds; the syntheses of perfluoroalkyl-substituted enamides, amides, thioamides, and hydrazones; and multicomponent perfluoroalkylation reactions will be studied. While there are a number of accomplished reports on organofluorination, we aim to provide an overview of radical-involved perfluoroalkylation reactions of aliphatic substrates, covering examples from 2018 to early 2023, as summarized in Tables 1–4.
中文翻译:

脂肪族底物的自由基全氟烷基化
将研究一系列具有方法学和合成幅度的脂肪族底物的全氟烷基化反应,并给出其应用示例和机理细节。记录了一系列氟烷基化反应的合成方法;特别是,激进方案在全氟烷基化方法中最为突出。为此,全氟烷基基团 R F (R F =C n F 2 n +1 , n> 1) 不饱和有机底物是获得氟烷基化支架最直接、最有效的方法之一。全氟烷基取代的乙烯基、炔基和烯丙基化合物的合成;全氟烷基取代的氢、碘和氧全氟烷基化烷烃和烯烃的合成;全氟烷基取代的羰基化合物的合成;全氟烷基取代的烯酰胺、酰胺、硫代酰胺和腙的合成;并将研究多组分全氟烷基化反应。虽然有许多关于有机氟化的已完成的报告,但我们的目标是提供脂肪族底物自由基参与的全氟烷基化反应的概述,涵盖 2018 年至 2023 年初的示例,如表 1-4 中总结。
更新日期:2023-05-25
中文翻译:

脂肪族底物的自由基全氟烷基化
将研究一系列具有方法学和合成幅度的脂肪族底物的全氟烷基化反应,并给出其应用示例和机理细节。记录了一系列氟烷基化反应的合成方法;特别是,激进方案在全氟烷基化方法中最为突出。为此,全氟烷基基团 R F (R F =C n F 2 n +1 , n> 1) 不饱和有机底物是获得氟烷基化支架最直接、最有效的方法之一。全氟烷基取代的乙烯基、炔基和烯丙基化合物的合成;全氟烷基取代的氢、碘和氧全氟烷基化烷烃和烯烃的合成;全氟烷基取代的羰基化合物的合成;全氟烷基取代的烯酰胺、酰胺、硫代酰胺和腙的合成;并将研究多组分全氟烷基化反应。虽然有许多关于有机氟化的已完成的报告,但我们的目标是提供脂肪族底物自由基参与的全氟烷基化反应的概述,涵盖 2018 年至 2023 年初的示例,如表 1-4 中总结。











































 京公网安备 11010802027423号
京公网安备 11010802027423号