当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Screening of Supported Metal Oxide Nanoclusters for Methane Activation: Insights into Homolytic versus Heterolytic C–H Bond Dissociation
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.jpclett.3c00863 Hieu A Doan 1 , Xijun Wang 1 , Randall Q Snurr 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.jpclett.3c00863 Hieu A Doan 1 , Xijun Wang 1 , Randall Q Snurr 1
Affiliation
|
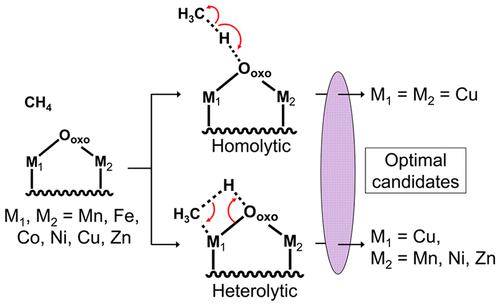
Since its discovery in zeolites, the [CuOCu]2+ motif has played an important role in our understanding of selective methane activation over supported metal oxide nanoclusters. Although there are two known C–H bond dissociation mechanisms, namely, homolytic and heterolytic cleavage, most computational studies on optimizing metal oxide nanoclusters for improved methane activation reactivity have focused only on the homolytic mechanism. In this work, both mechanisms were examined for a set of 21 mixed metal oxide complexes of the form of [M1OM2]2+ (M1 and M2 = Mn, Fe, Co, Ni, Cu, and Zn). Except for pure copper, heterolytic cleavage was found to be the dominant C–H bond activation pathway for all systems. Furthermore, mixed systems including [CuOMn]2+, [CuONi]2+, and [CuOZn]2+ are predicted to possess methane activation activity similar to pure [CuOCu]2+. These results suggest that both homolytic and heterolytic mechanisms should be considered in computing methane activation energies on supported metal oxide nanoclusters.
中文翻译:

用于甲烷活化的负载型金属氧化物纳米团簇的计算筛选:对均裂与杂裂 C-H 键解离的洞察
自从在沸石中发现 [CuOCu] 2+基序以来,它在我们理解负载型金属氧化物纳米团簇上的选择性甲烷活化方面发挥了重要作用。尽管有两种已知的 C-H 键解离机制,即均裂和异裂,但大多数关于优化金属氧化物纳米团簇以提高甲烷活化反应性的计算研究仅集中在均裂机制上。在这项工作中,针对一组 21 种形式为 [M 1 OM 2 ] 2+(M 1和 M 2= Mn、Fe、Co、Ni、Cu 和 Zn)。除纯铜外,发现异裂解是所有系统的主要 C-H 键激活途径。此外,包括[CuOMn] 2+、[CuONi] 2+和[CuOZn] 2+在内的混合系统预计具有类似于纯[CuOCu] 2+的甲烷活化活性。这些结果表明,在计算负载型金属氧化物纳米团簇上的甲烷活化能时,应同时考虑均裂和异裂机制。
更新日期:2023-05-24
中文翻译:

用于甲烷活化的负载型金属氧化物纳米团簇的计算筛选:对均裂与杂裂 C-H 键解离的洞察
自从在沸石中发现 [CuOCu] 2+基序以来,它在我们理解负载型金属氧化物纳米团簇上的选择性甲烷活化方面发挥了重要作用。尽管有两种已知的 C-H 键解离机制,即均裂和异裂,但大多数关于优化金属氧化物纳米团簇以提高甲烷活化反应性的计算研究仅集中在均裂机制上。在这项工作中,针对一组 21 种形式为 [M 1 OM 2 ] 2+(M 1和 M 2= Mn、Fe、Co、Ni、Cu 和 Zn)。除纯铜外,发现异裂解是所有系统的主要 C-H 键激活途径。此外,包括[CuOMn] 2+、[CuONi] 2+和[CuOZn] 2+在内的混合系统预计具有类似于纯[CuOCu] 2+的甲烷活化活性。这些结果表明,在计算负载型金属氧化物纳米团簇上的甲烷活化能时,应同时考虑均裂和异裂机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号