当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
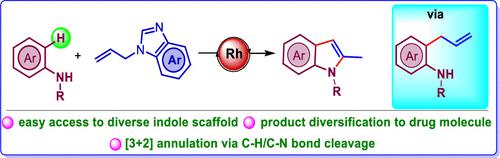
Rhodium-Catalyzed Synthesis of 2-Methylindoles via C–N Bond Cleavage of N-Allylbenzimidazole
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.joc.2c03048 Pragati Biswal 1, 2 , Tanmayee Nanda 1, 2 , Namrata Prusty 1, 2 , Smruti Ranjan Mohanty 1, 2 , Ponneri C Ravikumar 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.joc.2c03048 Pragati Biswal 1, 2 , Tanmayee Nanda 1, 2 , Namrata Prusty 1, 2 , Smruti Ranjan Mohanty 1, 2 , Ponneri C Ravikumar 1, 2
Affiliation
|
A rhodium-catalyzed oxidative C–H/N–H dehydrogenative [3 + 2] annulation strategy has been reported between anilines and N-allylbenzimidazole for the synthesis of 2-methylindole scaffolds. An N-allylbenzimidazole has been used as a 2C synthon for the synthesis of indole, and more importantly, this transformation involves the cleavage of the thermodynamically stable C–N bond of allylamine. Detailed mechanistic studies have been performed and a key intermediate was detected in HRMS. This transformation proceeds through a cascade of C(sp2)–H allylation followed by intramolecular cyclization.
中文翻译:

铑催化 N-烯丙基苯并咪唑 C-N 键断裂合成 2-甲基吲哚
据报道,苯胺和N-烯丙基苯并咪唑之间存在铑催化的氧化 C-H/N-H 脱氢 [3 + 2] 成环策略,用于合成 2-甲基吲哚支架。N-烯丙基苯并咪唑已被用作合成吲哚的2C合成子,更重要的是,这种转化涉及烯丙胺热力学稳定的C-N键的断裂。详细的机理研究已经完成,并在 HRMS 中检测到了关键的中间体。这种转化通过 C(sp 2 )–H 烯丙基化级联和随后的分子内环化进行。
更新日期:2023-05-24
中文翻译:

铑催化 N-烯丙基苯并咪唑 C-N 键断裂合成 2-甲基吲哚
据报道,苯胺和N-烯丙基苯并咪唑之间存在铑催化的氧化 C-H/N-H 脱氢 [3 + 2] 成环策略,用于合成 2-甲基吲哚支架。N-烯丙基苯并咪唑已被用作合成吲哚的2C合成子,更重要的是,这种转化涉及烯丙胺热力学稳定的C-N键的断裂。详细的机理研究已经完成,并在 HRMS 中检测到了关键的中间体。这种转化通过 C(sp 2 )–H 烯丙基化级联和随后的分子内环化进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号