当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
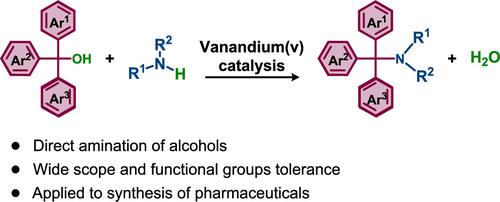
Direct Amination of α-Triaryl Alcohols via Vanadium Catalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acs.joc.3c00414 Jinglei Yang 1, 2 , Yun-Dong Wu 1, 2, 3 , Maoping Pu 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acs.joc.3c00414 Jinglei Yang 1, 2 , Yun-Dong Wu 1, 2, 3 , Maoping Pu 2
Affiliation
|
α-Triaryl amines have been used as pharmaceuticals and pharmaceutical intermediates for antifungal and anticancer applications. Current methods to synthesize such compounds require at least two steps, and no direct amination of tertiary alcohols has been reported. Herein, we disclose efficient catalytic conditions for the direct amination of α-triaryl alcohols to access α-triaryl amines. VO(OiPr)3, a commercially available reagent, has been identified as an effective catalyst for the direct amination of several α-triaryl alcohols. This process is scalable, as demonstrated by a gram-scale synthesis, and the reaction still works at as low as a 0.01 mol % catalyst loading with the turnover number reaching 3900. Moreover, commercial pharmaceuticals including clotrimazole and flutrimazole have been successfully prepared rapidly and efficiently using this newly developed method.
中文翻译:

钒催化α-三芳基醇的直接胺化
α-三芳基胺已被用作抗真菌和抗癌应用的药物和药物中间体。目前合成此类化合物的方法至少需要两步,并且没有报道过直接胺化叔醇。在此,我们公开了直接胺化 α-三芳基醇以获得 α-三芳基胺的有效催化条件。VO(O i Pr) 3,一种市售试剂,已被确定为几种α-三芳基醇直接胺化的有效催化剂。这个过程是可扩展的,正如克级合成所证明的那样,反应在低至 0.01 mol% 的催化剂负载下仍然有效,周转数达到 3900。此外,包括克霉唑和氟霉唑在内的商业药物已成功快速制备,有效地使用这种新开发的方法。
更新日期:2023-05-23
中文翻译:

钒催化α-三芳基醇的直接胺化
α-三芳基胺已被用作抗真菌和抗癌应用的药物和药物中间体。目前合成此类化合物的方法至少需要两步,并且没有报道过直接胺化叔醇。在此,我们公开了直接胺化 α-三芳基醇以获得 α-三芳基胺的有效催化条件。VO(O i Pr) 3,一种市售试剂,已被确定为几种α-三芳基醇直接胺化的有效催化剂。这个过程是可扩展的,正如克级合成所证明的那样,反应在低至 0.01 mol% 的催化剂负载下仍然有效,周转数达到 3900。此外,包括克霉唑和氟霉唑在内的商业药物已成功快速制备,有效地使用这种新开发的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号