当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correction to “Hydrolysis of Acetamide on Low-Index CeO2 Surfaces: Ceria as a Deamidation and General De-esterification Catalyst”
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c02062 Suman Bhasker-Ranganath , Ye Xu
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c02062 Suman Bhasker-Ranganath , Ye Xu
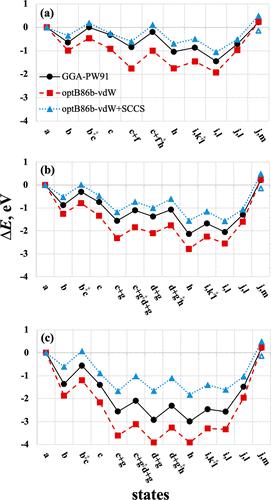
|
Figure 5 of the original article contains an error. The error was due to the use of gas-phase NH3 in the calculation of state (i,k‡l). Adsorbed NH3 should have been used instead because this state occurs prior to NH3 desorption. The error resulted in much larger activation barriers for OH attack on acetyl being depicted in the original Figure 5 for the three facets of ceria. They appear comparable to the barriers of acetic acid desorption, which is inconsistent with the conclusion that product desorption is rate-limiting in this reaction at ambient temperature. Figure 5. Minimum-energy reaction energy profiles for deamidation and hydrolysis of acetamide on (111), (110), and (100) facets (a-c) respectively, calculated using GGA-PW91 (black circles, VASP), optB86b-vdW (red squares, QE), and optB86b-vdW+SCCS (blue triangles, QE). The reaction states are (a) acetamide, water; (b) η1 acetamide; (b‡c) TS of nucleophilic attack by Olatt; (c) TI; (c+f) TI + H2O; (c+f‡h) TS of C–N scission + H2O; (c+g) TI + OH + H; (c+g‡d+g) TS of C–N scission + OH + H; (d+g) acetyl + NH2 + OH + H; (d+g‡h) TS of NH2 hydrogenation; (h) acetyl + NH3 + OH; (i,k‡l) NH3, TS of OH attack; (i,l) NH3, acetate + H; (j,l) NH3 desorbed; and (j,m) acetic acid desorbed. Labels correspond to those in Table 2. Hollow blue triangle in each panel corresponds to aqueous NH4+ and CH3COO–. The optB86b-vdW profile, when calculated using VASP (not shown), differs from QE (red squares) by 0.11 eV or less. The corrected version is shown below with the same caption as before. State (i,k‡l) is now revised to include NH3 adsorbed at infinite separation from the transition state k‡l. As can be seen, and as can be verified with entry k → l, Table 2 of the original article, the OH attack is not a kinetically relevant step in this reaction. All other states in Figure 5 have the correct energies. This article has not yet been cited by other publications. Figure 5. Minimum-energy reaction energy profiles for deamidation and hydrolysis of acetamide on (111), (110), and (100) facets (a-c) respectively, calculated using GGA-PW91 (black circles, VASP), optB86b-vdW (red squares, QE), and optB86b-vdW+SCCS (blue triangles, QE). The reaction states are (a) acetamide, water; (b) η1 acetamide; (b‡c) TS of nucleophilic attack by Olatt; (c) TI; (c+f) TI + H2O; (c+f‡h) TS of C–N scission + H2O; (c+g) TI + OH + H; (c+g‡d+g) TS of C–N scission + OH + H; (d+g) acetyl + NH2 + OH + H; (d+g‡h) TS of NH2 hydrogenation; (h) acetyl + NH3 + OH; (i,k‡l) NH3, TS of OH attack; (i,l) NH3, acetate + H; (j,l) NH3 desorbed; and (j,m) acetic acid desorbed. Labels correspond to those in Table 2. Hollow blue triangle in each panel corresponds to aqueous NH4+ and CH3COO–. The optB86b-vdW profile, when calculated using VASP (not shown), differs from QE (red squares) by 0.11 eV or less.
中文翻译:

更正“乙酰胺在低指数 CeO2 表面的水解:二氧化铈作为脱酰胺和一般脱酯催化剂”
原始文章的图 5 包含错误。错误是由于在计算状态 (i,k ‡ l)时使用了气相 NH 3 。应该使用吸附的 NH 3代替,因为这种状态发生在 NH 3之前解吸。该错误导致 OH 攻击乙酰基的激活障碍更大,如原始图 5 中所示,用于二氧化铈的三个方面。它们似乎与乙酸解吸的障碍相当,这与在环境温度下产物解吸在该反应中限速的结论不一致。图 5. 分别在 (111)、(110) 和 (100) 面 (ac) 上乙酰胺脱酰胺和水解的最小能量反应能量曲线,使用 GGA-PW91(黑色圆圈,VASP)、optB86b-vdW(红色方块,QE)和 optB86b-vdW+SCCS(蓝色三角形,QE)。反应状态是(a)乙酰胺,水;(b) η 1乙酰胺;(b ‡ c) O latt亲核攻击的 TS ;(c) 透明国际;(c+f) TI + H 2哦;(c+f ‡ h) C–N 断裂的 TS + H 2 O;(c+g) TI + OH + H;(c+g ‡ d+g) C–N 断裂的 TS + OH + H;(d+g)乙酰基+NH 2 +OH+H;(d+g ‡ h) NH 2加氢的TS;(h) 乙酰基 + NH 3 + OH;(i,k ‡ l) NH 3 , OH 攻击的 TS; (i,l) NH 3 , 醋酸盐 + H; (j,l)解吸的NH 3;和(j,m)解吸的乙酸。标签对应于表 2 中的标签。每个面板中的空心蓝色三角形对应于水性 NH 4 +和 CH 3 COO –. 使用 VASP(未显示)计算时,optB86b-vdW 配置文件与 QE(红色方块)相差 0.11 eV 或更小。更正后的版本如下所示,标题与之前相同。状态 (i,k ‡ l) 现在被修改为包括从过渡态 k ‡无限分离吸附的 NH 3湖。可以看出,并且可以通过原始文章的表 2 中的条目 k → l 进行验证,OH 攻击不是该反应中的动力学相关步骤。图 5 中的所有其他状态都具有正确的能量。这篇文章尚未被其他出版物引用。图 5. 分别在 (111)、(110) 和 (100) 面 (ac) 上乙酰胺脱酰胺和水解的最小能量反应能量曲线,使用 GGA-PW91(黑色圆圈,VASP)、optB86b-vdW(红色方块,QE)和 optB86b-vdW+SCCS(蓝色三角形,QE)。反应状态是(a)乙酰胺,水;(b) η 1乙酰胺;(b ‡ c) O latt亲核攻击的 TS ;(c) 透明国际;(c+f) TI + H 2 O;(c+f ‡ h) C–N 断裂的 TS + H2欧;(c+g) TI + OH + H;(c+g ‡ d+g) C–N 断裂的 TS + OH + H;(d+g)乙酰基+NH 2 +OH+H;(d+g ‡ h) NH 2加氢的TS;(h) 乙酰基 + NH 3 + OH;(i,k ‡ l) NH 3 , OH 攻击的 TS; (i,l) NH 3 , 醋酸盐 + H; (j,l)解吸的NH 3;和(j,m)解吸的乙酸。标签对应于表 2 中的标签。每个图中的空心蓝色三角形对应于水性 NH 4 +和 CH 3 COO –。使用 VASP(未显示)计算时,optB86b-vdW 配置文件与 QE(红色方块)相差 0.11 eV 或更小。
更新日期:2023-05-23
中文翻译:

更正“乙酰胺在低指数 CeO2 表面的水解:二氧化铈作为脱酰胺和一般脱酯催化剂”
原始文章的图 5 包含错误。错误是由于在计算状态 (i,k ‡ l)时使用了气相 NH 3 。应该使用吸附的 NH 3代替,因为这种状态发生在 NH 3之前解吸。该错误导致 OH 攻击乙酰基的激活障碍更大,如原始图 5 中所示,用于二氧化铈的三个方面。它们似乎与乙酸解吸的障碍相当,这与在环境温度下产物解吸在该反应中限速的结论不一致。图 5. 分别在 (111)、(110) 和 (100) 面 (ac) 上乙酰胺脱酰胺和水解的最小能量反应能量曲线,使用 GGA-PW91(黑色圆圈,VASP)、optB86b-vdW(红色方块,QE)和 optB86b-vdW+SCCS(蓝色三角形,QE)。反应状态是(a)乙酰胺,水;(b) η 1乙酰胺;(b ‡ c) O latt亲核攻击的 TS ;(c) 透明国际;(c+f) TI + H 2哦;(c+f ‡ h) C–N 断裂的 TS + H 2 O;(c+g) TI + OH + H;(c+g ‡ d+g) C–N 断裂的 TS + OH + H;(d+g)乙酰基+NH 2 +OH+H;(d+g ‡ h) NH 2加氢的TS;(h) 乙酰基 + NH 3 + OH;(i,k ‡ l) NH 3 , OH 攻击的 TS; (i,l) NH 3 , 醋酸盐 + H; (j,l)解吸的NH 3;和(j,m)解吸的乙酸。标签对应于表 2 中的标签。每个面板中的空心蓝色三角形对应于水性 NH 4 +和 CH 3 COO –. 使用 VASP(未显示)计算时,optB86b-vdW 配置文件与 QE(红色方块)相差 0.11 eV 或更小。更正后的版本如下所示,标题与之前相同。状态 (i,k ‡ l) 现在被修改为包括从过渡态 k ‡无限分离吸附的 NH 3湖。可以看出,并且可以通过原始文章的表 2 中的条目 k → l 进行验证,OH 攻击不是该反应中的动力学相关步骤。图 5 中的所有其他状态都具有正确的能量。这篇文章尚未被其他出版物引用。图 5. 分别在 (111)、(110) 和 (100) 面 (ac) 上乙酰胺脱酰胺和水解的最小能量反应能量曲线,使用 GGA-PW91(黑色圆圈,VASP)、optB86b-vdW(红色方块,QE)和 optB86b-vdW+SCCS(蓝色三角形,QE)。反应状态是(a)乙酰胺,水;(b) η 1乙酰胺;(b ‡ c) O latt亲核攻击的 TS ;(c) 透明国际;(c+f) TI + H 2 O;(c+f ‡ h) C–N 断裂的 TS + H2欧;(c+g) TI + OH + H;(c+g ‡ d+g) C–N 断裂的 TS + OH + H;(d+g)乙酰基+NH 2 +OH+H;(d+g ‡ h) NH 2加氢的TS;(h) 乙酰基 + NH 3 + OH;(i,k ‡ l) NH 3 , OH 攻击的 TS; (i,l) NH 3 , 醋酸盐 + H; (j,l)解吸的NH 3;和(j,m)解吸的乙酸。标签对应于表 2 中的标签。每个图中的空心蓝色三角形对应于水性 NH 4 +和 CH 3 COO –。使用 VASP(未显示)计算时,optB86b-vdW 配置文件与 QE(红色方块)相差 0.11 eV 或更小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号