当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Does Water-in-Salt Electrolyte Subdue Issues of Zn Batteries?
Advanced Materials ( IF 27.4 ) Pub Date : 2023-05-23 , DOI: 10.1002/adma.202300369 Ziyauddin Khan 1 , Divyaratan Kumar 1 , Xavier Crispin 1
Advanced Materials ( IF 27.4 ) Pub Date : 2023-05-23 , DOI: 10.1002/adma.202300369 Ziyauddin Khan 1 , Divyaratan Kumar 1 , Xavier Crispin 1
Affiliation
|
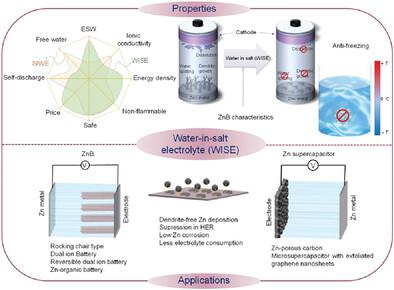
Zn-metal batteries (ZnBs) are safe and sustainable because of their operability in aqueous electrolytes, abundance of Zn, and recyclability. However, the thermodynamic instability of Zn metal in aqueous electrolytes is a major bottleneck for its commercialization. As such, Zn deposition (Zn2+ → Zn(s)) is continuously accompanied by the hydrogen evolution reaction (HER) (2H+ → H2) and dendritic growth that further accentuate the HER. Consequently, the local pH around the Zn electrode increases and promotes the formation of inactive and/or poorly conductive Zn passivation species (Zn + 2H2O → Zn(OH)2 + H2) on the Zn. This aggravates the consumption of Zn and electrolyte and degrades the performance of ZnB. To propel HER beyond its thermodynamic potential (0 V vs standard hydrogen electrode (SHE) at pH 0), the concept of water-in-salt-electrolyte (WISE) has been employed in ZnBs. Since the publication of the first article on WISE for ZnB in 2016, this research area has progressed continuously. Here, an overview and discussion on this promising research direction for accelerating the maturity of ZnBs is provided. The review briefly describes the current issues with conventional aqueous electrolyte in ZnBs, including a historic overview and basic understanding of WISE. Furthermore, the application scenarios of WISE in ZnBs are detailed, with the description of various key mechanisms (e.g., side reactions, Zn electrodeposition, anions or cations intercalation in metal oxide or graphite, and ion transport at low temperature).
中文翻译:

盐包水电解质能否解决锌电池的问题?
锌金属电池(ZnB)因其在水性电解质中的可操作性、丰富的锌和可回收性而安全且可持续。然而,金属锌在水性电解质中的热力学不稳定性是其商业化的主要瓶颈。因此,Zn沉积(Zn 2+ → Zn(s))持续伴随析氢反应(HER)(2H + → H 2)和进一步加剧HER的枝晶生长。因此,Zn 电极周围的局部 pH 值升高,并促进Zn 上不活泼和/或导电性差的 Zn 钝化物质 (Zn + 2H 2 O → Zn(OH) 2 + H 2 )的形成。这加剧了Zn和电解质的消耗并降低了ZnB的性能。为了推动 HER 超越其热力学势(0 V 相对于 pH 0 时的标准氢电极 (SHE)),ZnB 中采用了盐包水电解质 (WISE) 的概念。自2016年在WISE上发表第一篇关于ZnB的文章以来,该研究领域不断取得进展。本文对这一加速 ZnB 成熟的有前景的研究方向进行了概述和讨论。该综述简要描述了 ZnB 中传统水性电解质的当前问题,包括历史概述和对 WISE 的基本理解。此外,还详细介绍了WISE在ZnBs中的应用场景,并描述了各种关键机制(例如副反应、Zn电沉积、金属氧化物或石墨中的阴离子或阳离子插层以及低温下的离子传输)。
更新日期:2023-05-23
中文翻译:

盐包水电解质能否解决锌电池的问题?
锌金属电池(ZnB)因其在水性电解质中的可操作性、丰富的锌和可回收性而安全且可持续。然而,金属锌在水性电解质中的热力学不稳定性是其商业化的主要瓶颈。因此,Zn沉积(Zn 2+ → Zn(s))持续伴随析氢反应(HER)(2H + → H 2)和进一步加剧HER的枝晶生长。因此,Zn 电极周围的局部 pH 值升高,并促进Zn 上不活泼和/或导电性差的 Zn 钝化物质 (Zn + 2H 2 O → Zn(OH) 2 + H 2 )的形成。这加剧了Zn和电解质的消耗并降低了ZnB的性能。为了推动 HER 超越其热力学势(0 V 相对于 pH 0 时的标准氢电极 (SHE)),ZnB 中采用了盐包水电解质 (WISE) 的概念。自2016年在WISE上发表第一篇关于ZnB的文章以来,该研究领域不断取得进展。本文对这一加速 ZnB 成熟的有前景的研究方向进行了概述和讨论。该综述简要描述了 ZnB 中传统水性电解质的当前问题,包括历史概述和对 WISE 的基本理解。此外,还详细介绍了WISE在ZnBs中的应用场景,并描述了各种关键机制(例如副反应、Zn电沉积、金属氧化物或石墨中的阴离子或阳离子插层以及低温下的离子传输)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号