当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Cycle of the Bifunctional Enzyme Phosphoribosyl-ATP Pyrophosphohydrolase/Phosphoribosyl-AMP Cyclohydrolase
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c01111 Gemma Fisher 1 , Ennio Pečaver 1 , Benjamin J Read 1 , Susannah K Leese 1 , Erin Laing 1 , Alison L Dickson 1 , Clarissa M Czekster 1 , Rafael G da Silva 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c01111 Gemma Fisher 1 , Ennio Pečaver 1 , Benjamin J Read 1 , Susannah K Leese 1 , Erin Laing 1 , Alison L Dickson 1 , Clarissa M Czekster 1 , Rafael G da Silva 1
Affiliation
|
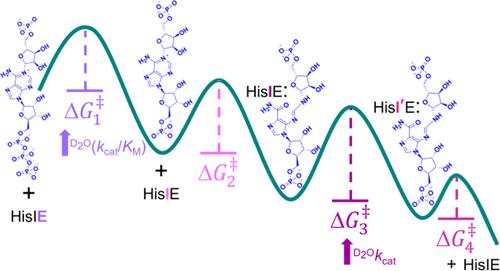
The bifunctional enzyme phosphoribosyl-ATP pyrophosphohydrolase/phosphoribosyl-AMP cyclohydrolase (HisIE) catalyzes the second and third steps of histidine biosynthesis: pyrophosphohydrolysis of N1-(5-phospho-β-D-ribosyl)-ATP (PRATP) to N1-(5-phospho-β-D-ribosyl)-AMP (PRAMP) and pyrophosphate in the C-terminal HisE-like domain, and cyclohydrolysis of PRAMP to N-(5′-phospho-D-ribosylformimino)-5-amino-1-(5″-phospho-D-ribosyl)-4-imidazolecarboxamide (ProFAR) in the N-terminal HisI-like domain. Here we use UV–VIS spectroscopy and LC–MS to show Acinetobacter baumannii putative HisIE produces ProFAR from PRATP. Employing an assay to detect pyrophosphate and another to detect ProFAR, we established the pyrophosphohydrolase reaction rate is higher than the overall reaction rate. We produced a truncated version of the enzyme-containing only the C-terminal (HisE) domain. This truncated HisIE was catalytically active, which allowed the synthesis of PRAMP, the substrate for the cyclohydrolysis reaction. PRAMP was kinetically competent for HisIE-catalyzed ProFAR production, demonstrating PRAMP can bind the HisI-like domain from bulk water, and suggesting that the cyclohydrolase reaction is rate-limiting for the overall bifunctional enzyme. The overall kcat increased with increasing pH, while the solvent deuterium kinetic isotope effect decreased at more basic pH but was still large at pH 7.5. The lack of solvent viscosity effects on kcat and kcat/KM ruled out diffusional steps limiting the rates of substrate binding and product release. Rapid kinetics with excess PRATP demonstrated a lag time followed by a burst in ProFAR formation. These observations are consistent with a rate-limiting unimolecular step involving a proton transfer following adenine ring opening. We synthesized N1-(5-phospho-β-D-ribosyl)-ADP (PRADP), which could not be processed by HisIE. PRADP inhibited HisIE-catalyzed ProFAR formation from PRATP but not from PRAMP, suggesting that it binds to the phosphohydrolase active site while still permitting unobstructed access of PRAMP to the cyclohydrolase active site. The kinetics data are incompatible with a build-up of PRAMP in bulk solvent, indicating HisIE catalysis involves preferential channeling of PRAMP, albeit not via a protein tunnel.
中文翻译:

双功能酶磷酸核糖-ATP 焦磷酸水解酶/磷酸核糖-AMP 环水解酶的催化循环
双功能酶磷酸核糖-ATP 焦磷酸水解酶/磷酸核糖-AMP 环水解酶 (HisIE) 催化组氨酸生物合成的第二步和第三步:N 1 -(5-磷酸-β-D-核糖基)-ATP (PRATP) 焦磷酸水解为N 1 - (5-磷酸-β-D-核糖基)-AMP (PRAMP) 和 C 端 HisE 样结构域中的焦磷酸盐,以及 PRAMP 环水解为N -(5'-磷酸-D-核糖基甲亚氨基)-5-氨基- N 末端 HisI 样结构域中的 1-(5″-磷酸-D-核糖基)-4-咪唑甲酰胺 (ProFAR)。在这里,我们使用 UV-VIS 光谱和 LC-MS 来显示鲍曼不动杆菌推定的 HisIE 从 PRATP 产生 ProFAR。采用检测焦磷酸盐的测定法和检测 ProFAR 的另一种测定法,我们确定焦磷酸水解酶反应速率高于总反应速率。我们制作了一种仅包含 C 末端 (HisE) 结构域的酶的截短版本。这种截短的 HisIE 具有催化活性,可以合成环水解反应的底物 PRAMP。PRAMP 在动力学上能够胜任 HisIE 催化的 ProFAR 生产,证明 PRAMP 可以结合大量水中的 HisI 样结构域,并表明环化水解酶反应是整个双功能酶的限速反应。整体k猫随着 pH 值的增加而增加,而溶剂氘动力学同位素效应在更碱性的 pH 值下降低,但在 pH 值 7.5 时仍然很大。溶剂粘度对k cat和k cat / K M的影响的缺乏排除了限制底物结合和产物释放速率的扩散步骤。具有过量 PRATP 的快速动力学表现出滞后时间,然后是 ProFAR 形成的爆发。这些观察结果与涉及腺嘌呤环打开后质子转移的限速单分子步骤一致。我们合成了N 1-(5-磷酸-β-D-核糖基)-ADP (PRADP),HisIE 无法处理。PRADP 抑制 HisIE 催化的 ProFAR 从 PRATP 而不是从 PRAMP 形成,表明它结合磷酸水解酶活性位点,同时仍然允许 PRAMP 畅通无阻地进入环水解酶活性位点。动力学数据与散装溶剂中 PRAMP 的积累不相容,表明 HisIE 催化涉及 PRAMP 的优先通道,尽管不是通过蛋白质隧道。
更新日期:2023-05-23
中文翻译:

双功能酶磷酸核糖-ATP 焦磷酸水解酶/磷酸核糖-AMP 环水解酶的催化循环
双功能酶磷酸核糖-ATP 焦磷酸水解酶/磷酸核糖-AMP 环水解酶 (HisIE) 催化组氨酸生物合成的第二步和第三步:N 1 -(5-磷酸-β-D-核糖基)-ATP (PRATP) 焦磷酸水解为N 1 - (5-磷酸-β-D-核糖基)-AMP (PRAMP) 和 C 端 HisE 样结构域中的焦磷酸盐,以及 PRAMP 环水解为N -(5'-磷酸-D-核糖基甲亚氨基)-5-氨基- N 末端 HisI 样结构域中的 1-(5″-磷酸-D-核糖基)-4-咪唑甲酰胺 (ProFAR)。在这里,我们使用 UV-VIS 光谱和 LC-MS 来显示鲍曼不动杆菌推定的 HisIE 从 PRATP 产生 ProFAR。采用检测焦磷酸盐的测定法和检测 ProFAR 的另一种测定法,我们确定焦磷酸水解酶反应速率高于总反应速率。我们制作了一种仅包含 C 末端 (HisE) 结构域的酶的截短版本。这种截短的 HisIE 具有催化活性,可以合成环水解反应的底物 PRAMP。PRAMP 在动力学上能够胜任 HisIE 催化的 ProFAR 生产,证明 PRAMP 可以结合大量水中的 HisI 样结构域,并表明环化水解酶反应是整个双功能酶的限速反应。整体k猫随着 pH 值的增加而增加,而溶剂氘动力学同位素效应在更碱性的 pH 值下降低,但在 pH 值 7.5 时仍然很大。溶剂粘度对k cat和k cat / K M的影响的缺乏排除了限制底物结合和产物释放速率的扩散步骤。具有过量 PRATP 的快速动力学表现出滞后时间,然后是 ProFAR 形成的爆发。这些观察结果与涉及腺嘌呤环打开后质子转移的限速单分子步骤一致。我们合成了N 1-(5-磷酸-β-D-核糖基)-ADP (PRADP),HisIE 无法处理。PRADP 抑制 HisIE 催化的 ProFAR 从 PRATP 而不是从 PRAMP 形成,表明它结合磷酸水解酶活性位点,同时仍然允许 PRAMP 畅通无阻地进入环水解酶活性位点。动力学数据与散装溶剂中 PRAMP 的积累不相容,表明 HisIE 催化涉及 PRAMP 的优先通道,尽管不是通过蛋白质隧道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号