当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Main Group Catalyzed Arene Borylation: Challenges and Opportunities
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c01668 Michael J Ingleson 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-23 , DOI: 10.1021/acscatal.3c01668 Michael J Ingleson 1
Affiliation
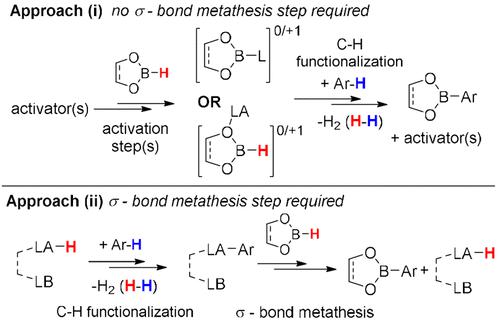
|
aLA = Lewis acid, LB = Lewis/Brønsted base. aThis excludes anion involvement for simplicity; with, e.g. [HB(C6F5) 3]−, more pathways are feasible. aCompounds 16 and inset 17 have not been reported to undergo σ-bond metathesis with hydroboranes to our knowledge. The manuscript was written through contributions of all authors. This project has received funding from the EPSRC (EP/V03829X/1) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 769599). Prof. S. P. Thomas and Dr. M. Bisai are thanked for useful discussions. This article references 44 other publications. For a recent review on metal-catalyzed C–H borylation, see: See (and references therein): For recent reviews, see: [H]+ indicates the proton is ligated by solvent, anion, and/or another base(s). For recent reviews see: For three select examples published at a similar time, see: For CatBH, see: For PinBH, see: Hence [PinB–L]+ species are weaker Lewis acids than [CatB–L]+ for a fixed L, see: ClCatBH, was required as CatBH itself underwent C–H borylation in initial studies. This article has not yet been cited by other publications. aLA = Lewis acid, LB = Lewis/Brønsted base. aThis excludes anion involvement for simplicity; with, e.g. [HB(C6F5) 3]−, more pathways are feasible. aCompounds 16 and inset 17 have not been reported to undergo σ-bond metathesis with hydroboranes to our knowledge. This article references 44 other publications. For a recent review on metal-catalyzed C–H borylation, see: See (and references therein): For recent reviews, see: [H]+ indicates the proton is ligated by solvent, anion, and/or another base(s). For recent reviews see: For three select examples published at a similar time, see: For CatBH, see: For PinBH, see: Hence [PinB–L]+ species are weaker Lewis acids than [CatB–L]+ for a fixed L, see: ClCatBH, was required as CatBH itself underwent C–H borylation in initial studies.
中文翻译:

主族催化芳烃硼酸化:挑战与机遇
a LA = 路易斯酸,LB = 路易斯/布朗斯特碱。a为简单起见,这不包括阴离子参与;例如 [HB(C 6 F 5 ) 3 ] -,更多途径是可行的。a化合物16和插图17据我们所知,还没有报道用氢硼烷进行 σ 键复分解。手稿是通过所有作者的贡献编写的。该项目已获得 EPSRC (EP/V03829X/1) 和欧洲研究理事会 (ERC) 在欧盟地平线 2020 研究与创新计划(资助协议号 769599)下的资助。感谢 SP Thomas 教授和 M. Bisai 博士进行了有益的讨论。本文引用了 44 篇其他出版物。有关金属催化的 C–H 硼化的最新评论,请参阅:请参阅(和其中的参考文献):有关最近的评论,请参阅:[H] +表示质子被溶剂、阴离子和/或其他碱基连接. 对于最近的评论,请参阅:对于同时发布的三个精选示例,请参阅:对于 CatBH,请参阅:对于 PinBH,请参阅:因此 [PinB–L] +对于固定的 L,物种是比 [CatB–L] +弱的路易斯酸,请参见: Cl CatBH,因为 CatBH 本身在初始研究中经历了 C–H 硼化。这篇文章尚未被其他出版物引用。a LA = 路易斯酸,LB = 路易斯/布朗斯特碱。a为简单起见,这不包括阴离子参与;例如 [HB(C 6 F 5 ) 3 ] -,更多途径是可行的。a化合物16和插图17据我们所知,还没有报道用氢硼烷进行 σ 键复分解。本文引用了 44 篇其他出版物。有关金属催化的 C–H 硼化的最新评论,请参阅:请参阅(和其中的参考文献):有关最近的评论,请参阅:[H] +表示质子被溶剂、阴离子和/或其他碱基连接. 对于最近的评论,请参阅:对于同时发布的三个精选示例,请参阅:对于 CatBH,请参阅:对于 PinBH,请参阅:因此 [PinB–L] + 物种是比 [ CatB –L] + 更弱的路易斯酸+对于固定的 L ,请参见:Cl CatBH,因为 CatBH 本身在初始研究中经历了 C-H 硼化。
更新日期:2023-05-23
中文翻译:

主族催化芳烃硼酸化:挑战与机遇
a LA = 路易斯酸,LB = 路易斯/布朗斯特碱。a为简单起见,这不包括阴离子参与;例如 [HB(C 6 F 5 ) 3 ] -,更多途径是可行的。a化合物16和插图17据我们所知,还没有报道用氢硼烷进行 σ 键复分解。手稿是通过所有作者的贡献编写的。该项目已获得 EPSRC (EP/V03829X/1) 和欧洲研究理事会 (ERC) 在欧盟地平线 2020 研究与创新计划(资助协议号 769599)下的资助。感谢 SP Thomas 教授和 M. Bisai 博士进行了有益的讨论。本文引用了 44 篇其他出版物。有关金属催化的 C–H 硼化的最新评论,请参阅:请参阅(和其中的参考文献):有关最近的评论,请参阅:[H] +表示质子被溶剂、阴离子和/或其他碱基连接. 对于最近的评论,请参阅:对于同时发布的三个精选示例,请参阅:对于 CatBH,请参阅:对于 PinBH,请参阅:因此 [PinB–L] +对于固定的 L,物种是比 [CatB–L] +弱的路易斯酸,请参见: Cl CatBH,因为 CatBH 本身在初始研究中经历了 C–H 硼化。这篇文章尚未被其他出版物引用。a LA = 路易斯酸,LB = 路易斯/布朗斯特碱。a为简单起见,这不包括阴离子参与;例如 [HB(C 6 F 5 ) 3 ] -,更多途径是可行的。a化合物16和插图17据我们所知,还没有报道用氢硼烷进行 σ 键复分解。本文引用了 44 篇其他出版物。有关金属催化的 C–H 硼化的最新评论,请参阅:请参阅(和其中的参考文献):有关最近的评论,请参阅:[H] +表示质子被溶剂、阴离子和/或其他碱基连接. 对于最近的评论,请参阅:对于同时发布的三个精选示例,请参阅:对于 CatBH,请参阅:对于 PinBH,请参阅:因此 [PinB–L] + 物种是比 [ CatB –L] + 更弱的路易斯酸+对于固定的 L ,请参见:Cl CatBH,因为 CatBH 本身在初始研究中经历了 C-H 硼化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号