当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
True Origin of Amide I Shifts Observed in Protein Spectra Obtained with Sum Frequency Generation Spectroscopy
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-05-22 , DOI: 10.1021/acs.jpclett.3c00391 Kuo-Yang Chiang 1 , Fumiki Matsumura 1 , Chun-Chieh Yu 1 , Daizong Qi 1 , Yuki Nagata 1 , Mischa Bonn 1 , Konrad Meister 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-05-22 , DOI: 10.1021/acs.jpclett.3c00391 Kuo-Yang Chiang 1 , Fumiki Matsumura 1 , Chun-Chieh Yu 1 , Daizong Qi 1 , Yuki Nagata 1 , Mischa Bonn 1 , Konrad Meister 1, 2
Affiliation
|
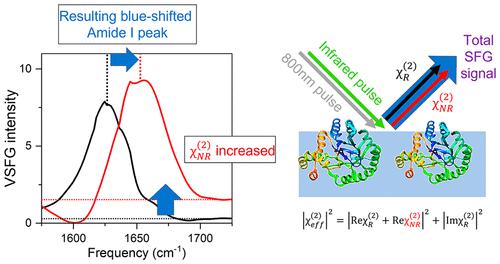
Accurate determination of protein structure at interfaces is critical for understanding protein interactions, which is directly relevant to a molecular-level understanding of interfacial proteins in biology and medicine. Vibrational sum frequency generation (VSFG) spectroscopy is often used for probing the protein amide I mode, which reports protein structures at interfaces. Observed peak shifts are attributed to conformational changes and often form the foundation of hypotheses explaining protein working mechanisms. Here, we investigate structurally diverse proteins using conventional and heterodyne-detected VSFG (HD-VSFG) spectroscopy as a function of solution pH. We reveal that blue-shifts of the amide I peak observed in conventional VSFG spectra upon lowering the pH are governed by the drastic change of the nonresonant contribution. Our results highlight that connecting changes in conventional VSFG spectra to conformational changes of interfacial proteins can be arbitrary, and that HD-VSFG measurements are required to draw unambiguous conclusions about structural changes in biomolecules.
中文翻译:

在用和频发生光谱法获得的蛋白质光谱中观察到的酰胺 I 位移的真正来源
准确测定界面处的蛋白质结构对于理解蛋白质相互作用至关重要,这与生物学和医学中界面蛋白质的分子水平理解直接相关。振动和频产生 (VSFG) 光谱通常用于探测蛋白质酰胺 I 模式,它报告界面处的蛋白质结构。观察到的峰移归因于构象变化,并且通常构成解释蛋白质工作机制的假设的基础。在这里,我们使用常规和外差检测 VSFG (HD-VSFG) 光谱作为溶液 pH 的函数来研究结构多样的蛋白质。我们揭示了在降低 pH 值时在传统 VSFG 光谱中观察到的酰胺 I 峰的蓝移是由非共振贡献的急剧变化决定的。
更新日期:2023-05-22
中文翻译:

在用和频发生光谱法获得的蛋白质光谱中观察到的酰胺 I 位移的真正来源
准确测定界面处的蛋白质结构对于理解蛋白质相互作用至关重要,这与生物学和医学中界面蛋白质的分子水平理解直接相关。振动和频产生 (VSFG) 光谱通常用于探测蛋白质酰胺 I 模式,它报告界面处的蛋白质结构。观察到的峰移归因于构象变化,并且通常构成解释蛋白质工作机制的假设的基础。在这里,我们使用常规和外差检测 VSFG (HD-VSFG) 光谱作为溶液 pH 的函数来研究结构多样的蛋白质。我们揭示了在降低 pH 值时在传统 VSFG 光谱中观察到的酰胺 I 峰的蓝移是由非共振贡献的急剧变化决定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号