Materials Today Chemistry ( IF 6.7 ) Pub Date : 2023-05-18 , DOI: 10.1016/j.mtchem.2023.101573 Y. Han , X. Mao , X. Yan , Q. Wu , H. Xu , Q. Fang , Y. Jia , X. Yao , Q. Li , A. Du
|
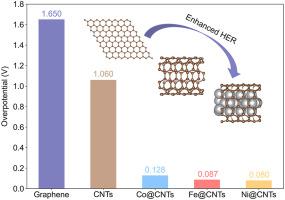
Carbon materials are widely used in various industrial applications due to their outstanding stability and robustness in diverse structures, yet it remains a revolutionary and challenging task in activating low-cost carbon materials for efficient catalysis. Herein, inspired by the successful experimental synthesis, we for the first-time exploited carbon nanotubes (CNTs) encapsulated transition metal (TM) atoms (TM@CNTs) for hydrogen evolution reaction (HER) using density functional theory (DFT) calculations. The Gibbs free energy of the H–C bond on the pristine CNTs is too positive, which prevents the adsorption of H atoms. However, TM@CNTs (TM = Fe, Co, Ni) have superior HER activity than those widely recognized Pt and MoS2 catalysts, benefiting from disrupting the π conjugations and activating the stable sp2 hybridizations among carbon atoms in CNTs. A new set of metal-free catalytic surfaces with strong HER activity have been developed. Meanwhile, the HER activity of graphene nanosheets loaded on the most ubiquitous facet (111) of TMs (TM@G, TM = Fe, Co, Ni) was also calculated. However, TM@G shows inferior HER activity than that of TM@CNTs, which is attributed to the large curvature of CNTs. These new findings manifest a universal strategy for carbon materials activation that will inspire the rational design of carbon-based electrocatalysts for efficient water splitting reaction.
中文翻译:

碳纳米管包封过渡金属用于高效析氢反应:3d 轨道和 π 键的耦合效应
碳材料由于其在不同结构中出色的稳定性和稳健性而被广泛用于各种工业应用,但激活低成本碳材料以进行高效催化仍然是一项革命性和挑战性的任务。在此,受成功实验合成的启发,我们首次利用密度泛函理论 (DFT) 计算利用碳纳米管 (CNT) 封装的过渡金属 (TM) 原子 (TM@CNT) 进行析氢反应 (HER)。原始碳纳米管上 H-C 键的吉布斯自由能太正,阻碍了 H 原子的吸附。然而,TM@CNTs (TM = Fe, Co, Ni) 具有优于那些广泛认可的 Pt 和 MoS 2催化剂的 HER 活性,这得益于破坏π共轭并激活CNT 中碳原子之间稳定的sp 2杂化。已经开发出一组具有强 HER 活性的新型无金属催化表面。同时,还计算了负载在 TM(TM@G,TM = Fe、Co、Ni)最普遍的小平面(111)上的石墨烯纳米片的 HER 活性。然而,TM@G 的 HER 活性低于 TM@CNT,这归因于 CNT 的大曲率。这些新发现表明了一种通用的碳材料活化策略,将激发碳基电催化剂的合理设计,以实现高效的水分解反应。























































 京公网安备 11010802027423号
京公网安备 11010802027423号