Journal of Hepatology ( IF 26.8 ) Pub Date : 2023-05-16 , DOI: 10.1016/j.jhep.2023.05.004 Samantha Schaeffer 1 , Barkha Gupta 1 , Anna-Line Calatayud 1 , Julien Calderaro 2 , Stefano Caruso 1 , Théo Z Hirsch 1 , Laura Pelletier 1 , Jessica Zucman-Rossi 3 , Sandra Rebouissou 1
|
Background & Aims
Recurrent somatic mutations of the RPS6KA3 gene encoding for the serine/threonine kinase RSK2 were identified in hepatocellular carcinomas (HCCs), suggesting its tumour-suppressive function. Our goal was to demonstrate the tumour suppressor role of RSK2 in the liver and investigate the functional consequences of its inactivation.
Methods
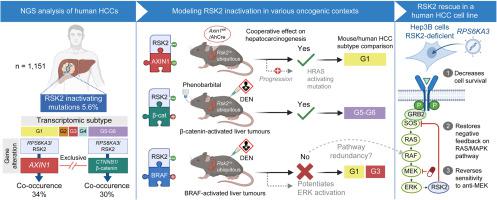
We analysed a series of 1,151 human HCCs for RSK2 mutations and 20 other driver genetic alterations. We then modelled RSK2 inactivation in mice in various mutational contexts recapitulating or not those naturally found in human HCC, using transgenic mice and liver-specific carcinogens. These models were monitored for liver tumour appearance and subjected to phenotypic and transcriptomic analyses. Functional consequences of RSK2 rescue were also investigated in a human RSK2-deficient HCC cell line.
Results
RSK2-inactivating mutations are specific to human HCC and frequently co-occur with AXIN1-inactivating or β-catenin-activating mutations. Modelling of these co-occurrences in mice showed a cooperative effect in promoting liver tumours with transcriptomic profiles recapitulating those of human HCCs. By contrast, there was no cooperation in liver tumour induction between RSK2 loss and BRAF-activating mutations chemically induced by diethylnitrosamine. In human liver cancer cells, we also showed that RSK2 inactivation confers some dependency to the activation of RAS/MAPK signalling that can be targeted by MEK inhibitors.
Conclusions
Our study demonstrates the tumour suppressor role of RSK2 and its specific synergistic effect in hepatocarcinogenesis when its loss of function is specifically combined with AXIN1 inactivation or β-catenin activation. Furthermore, we identified the RAS/MAPK pathway as a potential therapeutic target for RSK2-inactivated liver tumours.
Impact and Implications
This study demonstrated the tumour suppressor role of RSK2 in the liver and showed that its inactivation specifically synergises with AXIN1 inactivation or β-catenin activation to promote the development of HCC with similar transcriptomic profiles as found in humans. Furthermore, this study highlights that activation of the RAS/MAPK pathway is one of the key signalling pathways mediating the oncogenic effect of RSK2 inactivation that can be targeted with already available anti-MEK therapies.
中文翻译:

RSK2失活与AXIN1失活或β-catenin激活协同促进肝癌发生
背景与目标
在肝细胞癌 (HCC) 中发现了编码丝氨酸/苏氨酸激酶 RSK2 的RPS6KA3基因的反复体细胞突变,表明其具有肿瘤抑制功能。我们的目标是证明 RSK2 在肝脏中的肿瘤抑制作用并研究其失活的功能后果。
方法
我们分析了一系列 1,151 个人类 HCC 的 RSK2 突变和 20 个其他驱动基因改变。然后,我们使用转基因小鼠和肝脏特异性致癌物,在各种突变背景下对小鼠中的 RSK2 失活进行建模,这些突变背景是否重演人类 HCC 中自然发现的突变背景。监测这些模型的肝肿瘤外观并进行表型和转录组分析。还在人类 RSK2 缺陷型 HCC 细胞系中研究了 RSK2 拯救的功能后果。
结果
RSK2 失活突变是人类 HCC 特有的,并且经常与 AXIN1 失活或 β-连环蛋白激活突变同时发生。在小鼠中对这些同时发生的模型进行的建模表明,在促进肝脏肿瘤方面具有协同作用,其转录组学特征与人类肝癌的转录组学特征相似。相比之下,RSK2 缺失和二乙基亚硝胺化学诱导的 BRAF 激活突变在诱导肝肿瘤方面没有协同作用。在人类肝癌细胞中,我们还表明,RSK2 失活会导致对 RAS/MAPK 信号传导的激活产生一定依赖性,而 MEK 抑制剂可以靶向该信号传导。
结论
我们的研究证明了 RSK2 的肿瘤抑制作用,以及当其功能丧失与 AXIN1 失活或 β-catenin 激活特异性结合时,其在肝癌发生中的特异性协同作用。此外,我们确定 RAS/MAPK 通路是 RSK2 失活肝脏肿瘤的潜在治疗靶点。
影响和启示
这项研究证明了 RSK2 在肝脏中的肿瘤抑制作用,并表明其失活与 AXIN1 失活或 β-连环蛋白激活特异性协同作用,促进具有与人类相似的转录组谱的 HCC 的发展。此外,这项研究强调,RAS/MAPK 通路的激活是介导 RSK2 失活致癌作用的关键信号通路之一,可以用现有的抗 MEK 疗法来靶向这一作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号