Structure ( IF 5.7 ) Pub Date : 2023-05-15 , DOI: 10.1016/j.str.2023.04.011 Shiv K Sah-Teli 1 , Matyas Pinkas 2 , Mikko J Hynönen 1 , Sarah J Butcher 3 , Rik K Wierenga 1 , Jiri Novacek 2 , Rajaram Venkatesan 1
|
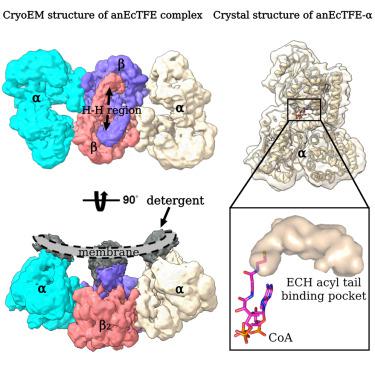
Facultative anaerobic bacteria such as Escherichia coli have two α2β2 heterotetrameric trifunctional enzymes (TFE), catalyzing the last three steps of the β-oxidation cycle: soluble aerobic TFE (EcTFE) and membrane-associated anaerobic TFE (anEcTFE), closely related to the human mitochondrial TFE (HsTFE). The cryo-EM structure of anEcTFE and crystal structures of anEcTFE-α show that the overall assembly of anEcTFE and HsTFE is similar. However, their membrane-binding properties differ considerably. The shorter A5-H7 and H8 regions of anEcTFE-α result in weaker α-β as well as α-membrane interactions, respectively. The protruding H-H region of anEcTFE-β is therefore more critical for membrane-association. Mutational studies also show that this region is important for the stability of the anEcTFE-β dimer and anEcTFE heterotetramer. The fatty acyl tail binding tunnel of the anEcTFE-α hydratase domain, as in HsTFE-α, is wider than in EcTFE-α, accommodating longer fatty acyl tails, in good agreement with their respective substrate specificities.
中文翻译:

大肠杆菌厌氧酶和人线粒体β-氧化三功能酶不同膜结合特性的结构基础
兼性厌氧菌如大肠杆菌有两个α 2 β 2异四聚体三功能酶 (TFE),催化 β-氧化循环的最后三个步骤:可溶性需氧 TFE (EcTFE) 和膜相关厌氧 TFE (anEcTFE),与人类线粒体 TFE (HsTFE) 密切相关。anEcTFE 的冷冻电镜结构和 anEcTFE-α 的晶体结构表明 anEcTFE 和 HsTFE 的整体组装是相似的。然而,它们的膜结合特性差异很大。anEcTFE-α 较短的 A5-H7 和 H8 区域分别导致较弱的 α-β 和 α-膜相互作用。因此,anEcTFE-β 突出的 HH 区域对于膜结合更为关键。突变研究还表明,该区域对于 anEcTFE-β 二聚体和 anEcTFE 异四聚体的稳定性很重要。anEcTFE-α 水合酶结构域的脂肪酰基尾结合隧道,如 HsTFE-α 中,



























 京公网安备 11010802027423号
京公网安备 11010802027423号