Structure ( IF 4.4 ) Pub Date : 2023-05-15 , DOI: 10.1016/j.str.2023.04.009 Spencer Cholak 1 , James W Saville 1 , Xing Zhu 1 , Alison M Berezuk 1 , Katharine S Tuttle 1 , Omid Haji-Ghassemi 2 , Francisco J Alvarado 3 , Filip Van Petegem 1 , Sriram Subramaniam 1
|
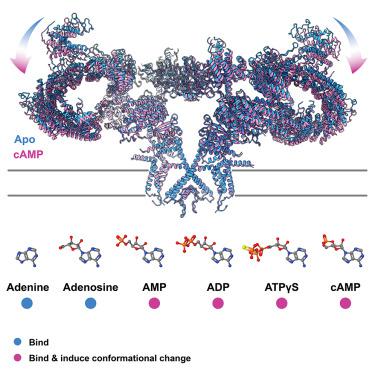
The coordinated release of Ca2+ from the sarcoplasmic reticulum (SR) is critical for excitation-contraction coupling. This release is facilitated by ryanodine receptors (RyRs) that are embedded in the SR membrane. In skeletal muscle, activity of RyR1 is regulated by metabolites such as ATP, which upon binding increase channel open probability (Po). To obtain structural insights into the mechanism of RyR1 priming by ATP, we determined several cryo-EM structures of RyR1 bound individually to ATP-γ-S, ADP, AMP, adenosine, adenine, and cAMP. We demonstrate that adenine and adenosine bind RyR1, but AMP is the smallest ATP derivative capable of inducing long-range (>170 Å) structural rearrangements associated with channel activation, establishing a structural basis for key binding site interactions that are the threshold for triggering quaternary structural changes. Our finding that cAMP also induces these structural changes and results in increased channel opening suggests its potential role as an endogenous modulator of RyR1 conductance.
中文翻译:

核苷酸衍生物对兰尼碱受体 RyR1 的变构调节
Ca 2+从肌浆网 (SR) 的协调释放对于兴奋-收缩耦合至关重要。这种释放是由嵌入 SR 膜中的兰尼碱受体 (RyR) 促进的。在骨骼肌中,RyR1 的活性受 ATP 等代谢物调节,结合后会增加通道开放概率 (P o )。为了深入了解 ATP 启动 RyR1 的机制,我们确定了 RyR1 的几种冷冻电镜结构,它们分别与 ATP-γ-S、ADP、AMP、腺苷、腺嘌呤和 cAMP 结合。我们证明腺嘌呤和腺苷结合 RyR1,但 AMP 是最小的 ATP 衍生物,能够诱导与通道激活相关的长程 (>170 Å) 结构重排,为关键结合位点相互作用奠定结构基础,这是触发四级反应的阈值结构性变化。我们发现 cAMP 也会诱导这些结构变化并导致通道开放增加,这表明它作为 RyR1 电导的内源调节剂的潜在作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号