当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
First-Generation Process Development of Napabucasin and Impurity Control Strategy
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-05-12 , DOI: 10.1021/acs.oprd.3c00001 Christopher E. Katz 1 , Bingidimi I. Mobele 2 , Jalal Haddad 3 , Yusuke Yamai 4 , Kazuki Hashimoto 4 , Yujiro Kiyoshima 5 , Justin Caserta 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2023-05-12 , DOI: 10.1021/acs.oprd.3c00001 Christopher E. Katz 1 , Bingidimi I. Mobele 2 , Jalal Haddad 3 , Yusuke Yamai 4 , Kazuki Hashimoto 4 , Yujiro Kiyoshima 5 , Justin Caserta 1
Affiliation
|
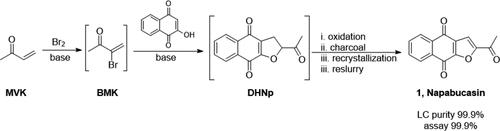
The manufacturing process for napabucasin described herein produced drug substance reliably on a scale up to 100 kg. The purification strategy employed was effective at removing process-related impurities and consistently afforded drug substance that met specifications. Below, we present an overview of the process development history and the impurity control strategy that was implemented to achieve robust manufacturing performance. Critical process parameters were identified, and process-related impurities and their proposed mechanisms of formation are presented.
中文翻译:

Napabucasin第一代工艺开发及杂质控制策略
本文所述的萘帕布卡星制造工艺可靠地以高达 100 千克的规模生产原料药。所采用的纯化策略可有效去除与工艺相关的杂质,并始终如一地提供符合规格的原料药。下面,我们概述了工艺开发历史和为实现稳健的制造性能而实施的杂质控制策略。确定了关键工艺参数,并介绍了与工艺相关的杂质及其建议的形成机制。
更新日期:2023-05-12
中文翻译:

Napabucasin第一代工艺开发及杂质控制策略
本文所述的萘帕布卡星制造工艺可靠地以高达 100 千克的规模生产原料药。所采用的纯化策略可有效去除与工艺相关的杂质,并始终如一地提供符合规格的原料药。下面,我们概述了工艺开发历史和为实现稳健的制造性能而实施的杂质控制策略。确定了关键工艺参数,并介绍了与工艺相关的杂质及其建议的形成机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号