当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Infusion of GMSCs relieves autoimmune arthritis by suppressing the externalization of neutrophil extracellular traps via PGE2-PKA-ERK axis
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2023-05-09 , DOI: 10.1016/j.jare.2023.05.001 Jun Zhao 1 , Yan Liu 2 , Xiaoyi Shi 2 , Junlong Dang 3 , Yu Liu 4 , Siwen Li 5 , Wei Cai 6 , Yuluan Hou 7 , Donglan Zeng 1 , Ye Chen 2 , Jia Yuan 7 , Yiding Xiong 1 , Wenbin Wu 8 , Peihong Cai 1 , Jingrong Chen 2 , Jianbo Sun 9 , Yiming Shao 9 , David D Brand 10 , Song Guo Zheng 11
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2023-05-09 , DOI: 10.1016/j.jare.2023.05.001 Jun Zhao 1 , Yan Liu 2 , Xiaoyi Shi 2 , Junlong Dang 3 , Yu Liu 4 , Siwen Li 5 , Wei Cai 6 , Yuluan Hou 7 , Donglan Zeng 1 , Ye Chen 2 , Jia Yuan 7 , Yiding Xiong 1 , Wenbin Wu 8 , Peihong Cai 1 , Jingrong Chen 2 , Jianbo Sun 9 , Yiming Shao 9 , David D Brand 10 , Song Guo Zheng 11
Affiliation
|
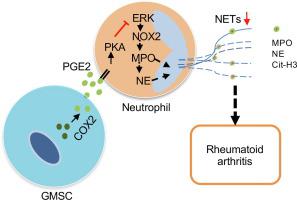
Rheumatoid arthritis (RA) is a systemic autoimmune disease with limited treatment success, characterized by chronic inflammation and progressive cartilage and bone destruction. Accumulating evidence has shown that neutrophil extracellular traps (NETs) released by activated neutrophils are important for initiating and perpetuating synovial inflammation and thereby could be a promising therapeutic target for RA. K/B × N serum transfer-induced arthritis (STIA) is a rapidly developed joint inflammatory model that somehow mimics the inflammatory response in patients with RA. Human gingival-derived mesenchymal stem cells (GMSCs) have been previously shown to possess immunosuppressive effects in arthritis and humanized animal models. However, it is unknown whether GMSCs can manage neutrophils in autoimmune arthritis. To evaluate whether infusion of GMSCs can alleviate RA by regulating neutrophils and NETs formation. If this is so, we will explore the underlying mechanism(s) in an animal model of inflammatory arthritis. The effects of GMSCs on RA were assessed by comparing the symptoms of the K/B × N serum transfer-induced arthritis (STIA) model administered either with GMSCs or with control cells. Phenotypes examined included clinical scores, rear ankle thickness, paw swelling, inflammation, synovial cell proliferation, and immune cell frequency. The regulation of GMSCs on NETs was examined through immunofluorescence and immunoblotting in GMSCs-infused STIA mice and in an co-culture system of neutrophils with GMSCs. The molecular mechanism(s) by which GMSCs regulate NETs was explored both and by silencing experiments. We found in this study that adoptive transfer of GMSCs into STIA mice significantly ameliorated experimental arthritis and reduced neutrophil infiltration and NET formation. studies also showed that GMSCs inhibited the generation of NETs in neutrophils. Subsequent investigations revealed that GMSCs secreted prostaglandin E2 (PGE2) to activate protein kinase A (PKA), which ultimately inhibited the downstream extracellular signal-regulated kinase (ERK) pathway that is essential for NET formation. Our results demonstrate that infusion of GMSCs can ameliorate inflammatory arthritis mainly by suppressing NET formation the PGE2-PKA-ERK signaling pathway. These findings further support the notion that the manipulation of GMSCs is a promising stem cell-based therapy for patients with RA and other autoimmune and inflammatory diseases.
中文翻译:

GMSCs 的输注通过 PGE2-PKA-ERK 轴抑制中性粒细胞胞外陷阱的外化来缓解自身免疫性关节炎
类风湿性关节炎(RA)是一种治疗效果有限的系统性自身免疫性疾病,其特征是慢性炎症和进行性软骨和骨质破坏。越来越多的证据表明,活化的中性粒细胞释放的中性粒细胞胞外陷阱(NET)对于滑膜炎症的引发和持续很重要,因此可能成为 RA 有希望的治疗靶点。 K/B × N 血清转移诱导性关节炎 (STIA) 是一种快速发展的关节炎症模型,以某种方式模拟 RA 患者的炎症反应。此前,人类牙龈来源的间充质干细胞(GMSC)已被证明在关节炎和人源化动物模型中具有免疫抑制作用。然而,GMSC 是否可以控制自身免疫性关节炎中的中性粒细胞尚不清楚。评估输注 GMSC 是否可以通过调节中性粒细胞和 NET 形成来缓解 RA。如果是这样,我们将探索炎症性关节炎动物模型的潜在机制。通过比较给予 GMSC 或对照细胞的 K/B × N 血清转移诱导性关节炎 (STIA) 模型的症状,评估 GMSC 对 RA 的影响。检查的表型包括临床评分、后踝厚度、爪肿胀、炎症、滑膜细胞增殖和免疫细胞频率。通过免疫荧光和免疫印迹在注入 GMSC 的 STIA 小鼠以及中性粒细胞与 GMSC 的共培养系统中检查 GMSC 对 NET 的调节。通过沉默实验探索了 GMSC 调节 NET 的分子机制。 我们在这项研究中发现,将 GMSC 过继转移到 STIA 小鼠体内可显着改善实验性关节炎,并减少中性粒细胞浸润和 NET 形成。研究还表明,GMSC 抑制中性粒细胞中 NET 的生成。随后的研究表明,GMSCs 分泌前列腺素 E2 (PGE2) 来激活蛋白激酶 A (PKA),最终抑制下游细胞外信号调节激酶 (ERK) 通路,该通路对于 NET 形成至关重要。我们的结果表明,输注 GMSC 可以主要通过抑制 PGE2-PKA-ERK 信号通路中 NET 的形成来改善炎症性关节炎。这些发现进一步支持了这样的观点,即对于 RA 和其他自身免疫性疾病和炎症性疾病患者来说,GMSC 的操作是一种有前途的基于干细胞的疗法。
更新日期:2023-05-09
中文翻译:

GMSCs 的输注通过 PGE2-PKA-ERK 轴抑制中性粒细胞胞外陷阱的外化来缓解自身免疫性关节炎
类风湿性关节炎(RA)是一种治疗效果有限的系统性自身免疫性疾病,其特征是慢性炎症和进行性软骨和骨质破坏。越来越多的证据表明,活化的中性粒细胞释放的中性粒细胞胞外陷阱(NET)对于滑膜炎症的引发和持续很重要,因此可能成为 RA 有希望的治疗靶点。 K/B × N 血清转移诱导性关节炎 (STIA) 是一种快速发展的关节炎症模型,以某种方式模拟 RA 患者的炎症反应。此前,人类牙龈来源的间充质干细胞(GMSC)已被证明在关节炎和人源化动物模型中具有免疫抑制作用。然而,GMSC 是否可以控制自身免疫性关节炎中的中性粒细胞尚不清楚。评估输注 GMSC 是否可以通过调节中性粒细胞和 NET 形成来缓解 RA。如果是这样,我们将探索炎症性关节炎动物模型的潜在机制。通过比较给予 GMSC 或对照细胞的 K/B × N 血清转移诱导性关节炎 (STIA) 模型的症状,评估 GMSC 对 RA 的影响。检查的表型包括临床评分、后踝厚度、爪肿胀、炎症、滑膜细胞增殖和免疫细胞频率。通过免疫荧光和免疫印迹在注入 GMSC 的 STIA 小鼠以及中性粒细胞与 GMSC 的共培养系统中检查 GMSC 对 NET 的调节。通过沉默实验探索了 GMSC 调节 NET 的分子机制。 我们在这项研究中发现,将 GMSC 过继转移到 STIA 小鼠体内可显着改善实验性关节炎,并减少中性粒细胞浸润和 NET 形成。研究还表明,GMSC 抑制中性粒细胞中 NET 的生成。随后的研究表明,GMSCs 分泌前列腺素 E2 (PGE2) 来激活蛋白激酶 A (PKA),最终抑制下游细胞外信号调节激酶 (ERK) 通路,该通路对于 NET 形成至关重要。我们的结果表明,输注 GMSC 可以主要通过抑制 PGE2-PKA-ERK 信号通路中 NET 的形成来改善炎症性关节炎。这些发现进一步支持了这样的观点,即对于 RA 和其他自身免疫性疾病和炎症性疾病患者来说,GMSC 的操作是一种有前途的基于干细胞的疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号