Cell ( IF 45.5 ) Pub Date : 2023-05-04 , DOI: 10.1016/j.cell.2023.04.018 Jak Grimes 1 , Zsombor Koszegi 1 , Yann Lanoiselée 1 , Tamara Miljus 1 , Shannon L O'Brien 1 , Tomasz M Stepniewski 2 , Brian Medel-Lacruz 2 , Mithu Baidya 3 , Maria Makarova 4 , Ravi Mistry 1 , Joëlle Goulding 5 , Julia Drube 6 , Carsten Hoffmann 6 , Dylan M Owen 7 , Arun K Shukla 3 , Jana Selent 2 , Stephen J Hill 5 , Davide Calebiro 1
|
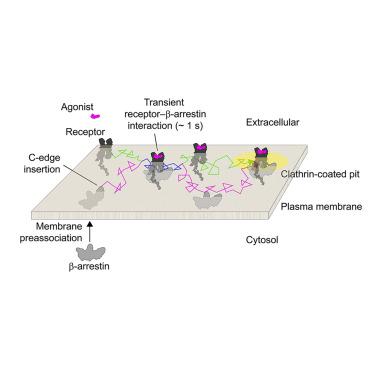
β-arrestin plays a key role in G protein-coupled receptor (GPCR) signaling and desensitization. Despite recent structural advances, the mechanisms that govern receptor-β-arrestin interactions at the plasma membrane of living cells remain elusive. Here, we combine single-molecule microscopy with molecular dynamics simulations to dissect the complex sequence of events involved in β-arrestin interactions with both receptors and the lipid bilayer. Unexpectedly, our results reveal that β-arrestin spontaneously inserts into the lipid bilayer and transiently interacts with receptors via lateral diffusion on the plasma membrane. Moreover, they indicate that, following receptor interaction, the plasma membrane stabilizes β-arrestin in a longer-lived, membrane-bound state, allowing it to diffuse to clathrin-coated pits separately from the activating receptor. These results expand our current understanding of β-arrestin function at the plasma membrane, revealing a critical role for β-arrestin preassociation with the lipid bilayer in facilitating its interactions with receptors and subsequent activation.
中文翻译:

质膜预缔合驱动 β-arrestin 与受体偶联并激活
β-arrestin 在 G 蛋白偶联受体 (GPCR) 信号传导和脱敏中发挥关键作用。尽管最近在结构上取得了进展,但控制活细胞质膜上受体-β-抑制蛋白相互作用的机制仍然难以捉摸。在这里,我们将单分子显微镜与分子动力学模拟相结合,剖析β-抑制蛋白与受体和脂质双层相互作用所涉及的复杂事件序列。出乎意料的是,我们的结果表明,β-抑制蛋白自发插入脂质双层,并通过质膜上的横向扩散与受体短暂相互作用。此外,他们表明,在受体相互作用后,质膜将β-抑制蛋白稳定在寿命较长的膜结合状态,使其能够与激活受体分开扩散到网格蛋白包被的凹坑中。这些结果扩展了我们目前对质膜上 β-arrestin 功能的理解,揭示了 β-arrestin 与脂质双层的预结合在促进其与受体相互作用和随后的激活中的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号