当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonaqueous Electrochemistry of Uranium Complexes: A Guide to Structure–Reactivity Tuning
Chemical Reviews ( IF 51.4 ) Pub Date : 2023-05-03 , DOI: 10.1021/acs.chemrev.2c00903 Judith Riedhammer 1 , Dominik P Halter 2 , Karsten Meyer 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2023-05-03 , DOI: 10.1021/acs.chemrev.2c00903 Judith Riedhammer 1 , Dominik P Halter 2 , Karsten Meyer 1
Affiliation
|
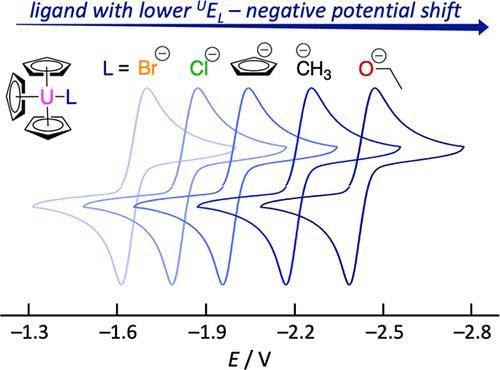
Uranium complexes can be stabilized in a wide range of oxidation states, ranging from UII to UVI and a very recent example of a UI complex. This review provides a comprehensive summary of electrochemistry data reported on uranium complexes in nonaqueous electrolyte, to serve as a clear point of reference for newly synthesized compounds, and to evaluate how different ligand environments influence experimentally observed electrochemical redox potentials. Data for over 200 uranium compounds are reported, together with a detailed discussion of trends observed across larger series of complexes in response to ligand field variations. In analogy to the traditional Lever parameter, we utilized the data to derive a new uranium-specific set of ligand field parameters UEL(L) that more accurately represent metal–ligand bonding situations than previously existing transition metal derived parameters. Exemplarily, we demonstrate UEL(L) parameters to be useful for the prediction of structure–reactivity correlations in order to activate specific substrate targets.
中文翻译:

铀配合物的非水电化学:结构-反应性调节指南
铀配合物可以在多种氧化态下稳定,从 U II到 U VI以及最近的 U I配合物的例子。本综述对非水电解质中铀配合物的电化学数据进行了全面总结,作为新合成化合物的明确参考点,并评估不同配体环境如何影响实验观察到的电化学氧化还原电位。报告了 200 多种铀化合物的数据,并详细讨论了在较大系列配合物中观察到的响应配体场变化的趋势。与传统的杠杆参数类似,我们利用这些数据推导出一组新的铀特异性配体场参数U E L (L) 比以前存在的过渡金属衍生参数更准确地表示金属-配体键合情况。举例来说,我们证明U E L (L) 参数可用于预测结构-反应性相关性,以激活特定的底物目标。
更新日期:2023-05-03
中文翻译:

铀配合物的非水电化学:结构-反应性调节指南
铀配合物可以在多种氧化态下稳定,从 U II到 U VI以及最近的 U I配合物的例子。本综述对非水电解质中铀配合物的电化学数据进行了全面总结,作为新合成化合物的明确参考点,并评估不同配体环境如何影响实验观察到的电化学氧化还原电位。报告了 200 多种铀化合物的数据,并详细讨论了在较大系列配合物中观察到的响应配体场变化的趋势。与传统的杠杆参数类似,我们利用这些数据推导出一组新的铀特异性配体场参数U E L (L) 比以前存在的过渡金属衍生参数更准确地表示金属-配体键合情况。举例来说,我们证明U E L (L) 参数可用于预测结构-反应性相关性,以激活特定的底物目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号