当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Remdesivir by Direct Lithiation of Pyrrolo[2,1-f][1,2,4]triazine Derivatives Enabled C-Glycosylation
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2023-04-28 , DOI: 10.1002/ejoc.202300197 Chao Xu 1 , Yuhan Hu 2 , Yong He 3 , Zhigang Hu 3 , Shikuo Li 4 , Hui Zhang 5 , Fangzhi Huang 6 , Fenglian Zhang 2 , yuelei Chen 7
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2023-04-28 , DOI: 10.1002/ejoc.202300197 Chao Xu 1 , Yuhan Hu 2 , Yong He 3 , Zhigang Hu 3 , Shikuo Li 4 , Hui Zhang 5 , Fangzhi Huang 6 , Fenglian Zhang 2 , yuelei Chen 7
Affiliation
|
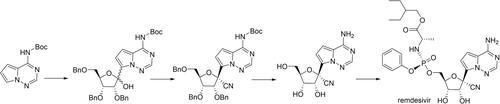
A direct and regioselective lithiation was realized on N-Boc-pyrrolo[2,1-f][1,2,4]triazine, simply utilizing the distinct sigma-acidity on C-9. Starting from this enabling discovery, C-glycoside formation, cyanation, and global deprotection steps constitute one of the most efficient syntheses of GS-441524. Furthermore, transient protection was applied on GS-441524 with phenyl boronic acid, which facilitated a powerful one-pot synthesis of remdesivir.
中文翻译:

吡咯并[2,1-f][1,2,4]三嗪衍生物直接锂化合成瑞德西韦 C-糖基化
只需利用 C-9 上独特的 σ 酸性,即可在N -Boc-吡咯并[2,1-f][1,2,4]三嗪上实现直接和区域选择性的锂化。从这一发现出发,C-糖苷形成、氰化和整体脱保护步骤构成了 GS-441524 最有效的合成方法之一。此外,用苯基硼酸对 GS-441524 进行瞬时保护,促进了瑞德西韦的强大一锅合成。
更新日期:2023-04-28
中文翻译:

吡咯并[2,1-f][1,2,4]三嗪衍生物直接锂化合成瑞德西韦 C-糖基化
只需利用 C-9 上独特的 σ 酸性,即可在N -Boc-吡咯并[2,1-f][1,2,4]三嗪上实现直接和区域选择性的锂化。从这一发现出发,C-糖苷形成、氰化和整体脱保护步骤构成了 GS-441524 最有效的合成方法之一。此外,用苯基硼酸对 GS-441524 进行瞬时保护,促进了瑞德西韦的强大一锅合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号