Journal of Hepatology ( IF 26.8 ) Pub Date : 2023-04-26 , DOI: 10.1016/j.jhep.2023.04.019 Yumi Sheehan 1 , Evan B Cunningham 1 , Amanda Cochrane 2 , Marianne Byrne 1 , Tracey Brown 2 , Colette McGrath 2 , Lise Lafferty 3 , Nicodemus Tedla 4 , Gregory J Dore 1 , Andrew R Lloyd 1 , Jason Grebely 1
|
Background & Aims
Prisons are key venues for scaling-up hepatitis C virus (HCV) testing and treatment. Complex clinical pathways and frequent movements of people in prison remain barriers to HCV care. This study evaluated the impact of a ‘one-stop-shop’ point-of-care HCV RNA testing intervention on treatment uptake compared with standard of care among people recently incarcerated in Australia.
Methods
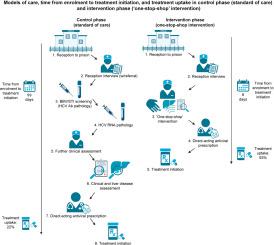
PIVOT was a prospective, non-concurrent, controlled study comparing HCV treatment uptake during ‘standard of care’ (n = 239; November 2019–May 2020) and a ‘one-stop-shop’ intervention (n = 301; June 2020–April 2021) in one reception prison in Australia. The primary endpoint was uptake of direct-acting antiviral treatment at 12 weeks from enrolment. Secondary outcomes included the time taken from enrolment to each stage in the care cascade.
Results
A total of 540 male participants were enrolled. Median age (29 vs. 28 years) and history of injecting drug use (48% vs. 42%) were similar between standard of care and intervention phases. Among people diagnosed with current HCV infection (n = 18/63 in the standard of care phase vs. n = 30/298 in the intervention phase), the proportion initiating direct-acting antiviral treatment within 12 weeks from enrolment in the intervention phase was higher (93% [95% CI 0.78–0.99] vs. 22% [95% CI 0.64–0.48]; p <0.001), and the median time to treatment initiation was shorter (6 days [IQR 5–7] vs. 99 days [IQR 57–127]; p <0.001) compared to standard of care.
Conclusions
The ‘one-stop-shop’ intervention enhanced treatment uptake and reduced time to treatment initiation among people recently incarcerated in Australia, thereby overcoming key barriers to treatment scale-up in the prison sector.
Impact and implications
This study provides important insights for policymakers regarding optimal HCV testing and treatment pathways for people newly incarcerated in prisons. The findings will improve health outcomes in people in prison with chronic HCV infection by increasing testing and treatment, thereby reducing infections, liver-related morbidity/mortality, and comorbidities. The findings will change clinical practice, clinical guidelines, and international guidance, and will inform future research and national and regional strategies, in particular regarding point-of-care testing, which is being rapidly scaled-up in various settings globally. The economic impact will likely include health budget savings resulting from reduced negative health outcomes relating to HCV, and health system efficiencies resulting from the introduction of simplified models of care.
Clinical Trials Registration
This study is registered at Clinicaltrials.gov (NCT04809246).
中文翻译:

“一站式”护理点丙型肝炎 RNA 检测干预措施,以提高收容监狱的治疗接受率:PIVOT 研究
背景与目标
监狱是扩大丙型肝炎病毒(HCV)检测和治疗规模的关键场所。复杂的临床路径和监狱人员的频繁流动仍然是丙肝治疗的障碍。这项研究评估了“一站式”护理点 HCV RNA 检测干预措施与澳大利亚最近被监禁者的治疗标准相比对治疗的影响。
方法
PIVOT 是一项前瞻性、非同步、对照研究,比较“标准护理”(n = 239;2019 年 11 月至 2020 年 5 月)和“一站式”干预(n = 301;2020 年 6 月至 2020 年 5 月)期间 HCV 治疗的接受情况2021 年 4 月)在澳大利亚的一所接待监狱。主要终点是入组后 12 周时接受直接作用抗病毒治疗。次要结果包括从登记到护理级联每个阶段所需的时间。
结果
共有 540 名男性参与者入组。标准护理和干预阶段之间的中位年龄(29 岁与28 岁)和注射吸毒史(48%与42%)相似。在诊断为当前 HCV 感染的人群中(标准护理阶段 n = 18/63 vs干预阶段 n = 30/298),在干预阶段入组后 12 周内开始直接作用抗病毒治疗的比例为较高(93% [95% CI 0.78–0.99] vs. 22% [95% CI 0.64–0.48];p <0.001),并且开始治疗的中位时间较短(6 天 [IQR 5–7] vs 6 天)。 99 天 [IQR 57–127];p <0.001)与标准护理相比。
结论
“一站式”干预措施提高了澳大利亚最近被监禁者的治疗接受率并缩短了开始治疗的时间,从而克服了监狱部门扩大治疗规模的主要障碍。
影响和影响
这项研究为政策制定者提供了关于新入狱人员的最佳丙型肝炎病毒检测和治疗途径的重要见解。研究结果将通过增加检测和治疗来改善慢性丙型肝炎感染监狱人员的健康状况,从而减少感染、肝脏相关发病率/死亡率和合并症。这些发现将改变临床实践、临床指南和国际指导,并将为未来的研究以及国家和地区战略提供信息,特别是在全球各种环境中迅速扩大的即时检测方面。经济影响可能包括因丙肝相关负面健康结果减少而节省的卫生预算,以及因引入简化护理模式而提高卫生系统效率。
临床试验注册
本研究已在ClinicalTrials.gov上注册(NCT04809246)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号