Journal of Advanced Research ( IF 11.4 ) Pub Date : 2023-04-20 , DOI: 10.1016/j.jare.2023.04.013 Yipin Lv 1 , Wenqing Tian 2 , Yongsheng Teng 3 , Pan Wang 3 , Yongliang Zhao 4 , Zhengyan Li 4 , Shanhong Tang 5 , Weisan Chen 6 , Rui Xie 7 , Muhan Lü 8 , Yuan Zhuang 9
|
Introduction
In solid tumors, regulatory T cell (Treg) and mast cell perform different roles depending on the microenvironment. Nevertheless, mast cell and Treg-mediated interactions in gastric cancer (GC) are unclear, as are their regulation, function, and clinical significance.
Objective
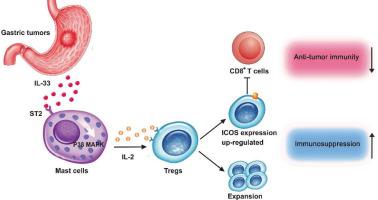
The present study demonstrated the mechanism of tumor-infiltrating mast cells stimulating ICOS+ regulatory T cells via the IL-33/IL-2 axis to promote the growth of gastric cancer.
Methods
Analyses of 98 patients with GC were conducted to examine mast cell counts, ICOS+ Tregs, and the levels of IL-33 or IL-2. Isolated ICOS+ Treg and CD8+ T cell were stimulated, cultured and tested for their functional abilities in vitro and in vivo.
Results
GC patients exhibited a significantly more production of IL-33 in tumors. Mast cell stimulated by tumor-derived IL-33 exhibited a prolonged lifespan through IL-33 mediated inhibition of apoptosis. Moreover, mast cells stimulated by tumor-derived IL-33 secreted IL-2, which induced Treg expansion. These inducible Tregs displayed an activated immunosuppressive phenotype with positive expression for the inducible T cell co-stimulator (ICOS). In vitro, IL-2 from IL-33-stimulated mast cells induced increased numbers of ICOS+ Tregs with increased immunosuppressive activity against proliferation and effector function of CD8+ T cell. In vivo, ICOS+ Tregs were treated with anti-IL-2 neutralizing antibody followed by co-injection with CD8+ T cells in GC mouse model, which showed an increased CD8+ T cell infiltration and effector molecules production, meanwhile tumor growth and progression were inhibited. Besides, reduction in GC patient survival was associated with tumor-derived ICOS+ Tregs.
Conclusion
Our results highlight a crosstalk between GC-infiltrating mast cells and ICOS+ Tregs and provide a novel mechanism describing ICOS+ Treg expansion and induction by an IL-33/mast cell/IL-2 signaling axis in GC, and also provide functional evidence that the modulation of this immunosuppressive pathway can attenuate GC-mediated immune tolerance.
中文翻译:

肿瘤浸润肥大细胞通过 IL-33 和 IL-2 轴刺激 ICOS+ 调节性 T 细胞,促进胃癌进展
介绍
在实体瘤中,调节性 T 细胞 (Treg) 和肥大细胞根据微环境发挥不同的作用。然而,胃癌 (GC) 中肥大细胞和 Treg 介导的相互作用以及它们的调节、功能和临床意义尚不清楚。
客观的
本研究证明了肿瘤浸润肥大细胞通过IL-33/IL-2轴刺激ICOS +调节性T细胞促进胃癌生长的机制。
方法
对 98 名 GC 患者进行了分析,以检查肥大细胞计数、ICOS + Tregs 以及 IL-33 或 IL-2 水平。对分离的 ICOS + Treg 和 CD8 + T 细胞进行刺激、培养并测试其体外和体内功能。
结果
胃癌患者的肿瘤中 IL-33 的产生显着增加。肿瘤源性 IL-33 刺激的肥大细胞通过 IL-33 介导的细胞凋亡抑制表现出延长的寿命。此外,肿瘤来源的 IL-33 刺激肥大细胞分泌 IL-2,从而诱导 Treg 扩增。这些可诱导的 Tregs 显示出激活的免疫抑制表型,并可诱导 T 细胞共刺激因子 (ICOS) 呈阳性表达。在体外,来自 IL-33 刺激的肥大细胞的 IL-2 诱导 ICOS + Tregs 数量增加,并增加针对 CD8 + T 细胞增殖和效应功能的免疫抑制活性。在体内,GC小鼠模型中,用抗IL-2中和抗体处理ICOS + Tregs,然后与CD8 + T细胞共注射,结果显示CD8 + T细胞浸润和效应分子产生增加,同时肿瘤生长和进展被抑制。此外,GC 患者生存率的降低与肿瘤衍生的 ICOS + Tregs 相关。
结论
我们的结果强调了 GC 浸润性肥大细胞和 ICOS + Tregs 之间的串扰,并提供了一种描述 GC 中 IL-33/肥大细胞/IL-2 信号轴诱导的 ICOS + Treg 扩增和诱导的新机制,并且还提供了功能证据:这种免疫抑制途径的调节可以减弱 GC 介导的免疫耐受。











































 京公网安备 11010802027423号
京公网安备 11010802027423号