当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Consideration of the Extent That Tertiary Amines Can Form N-Nitroso Dialkylamines in Pharmaceutical Products
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-04-19 , DOI: 10.1021/acs.oprd.3c00073 Ian W. Ashworth 1 , Timothy Curran 2 , Olivier Dirat 3 , Jinjian Zheng 4 , Matthew Whiting 5 , Daniel Lee 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-04-19 , DOI: 10.1021/acs.oprd.3c00073 Ian W. Ashworth 1 , Timothy Curran 2 , Olivier Dirat 3 , Jinjian Zheng 4 , Matthew Whiting 5 , Daniel Lee 4
Affiliation
|
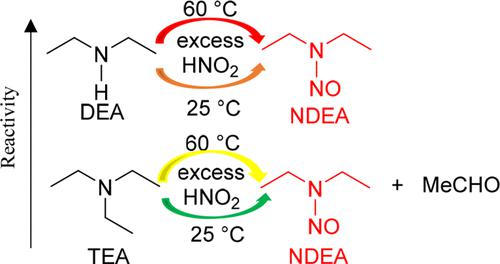
Most secondary amines have the potential to undergo nitrosation in the presence of nitrite under certain conditions, particularly at low pH, to generate N-nitrosamines. Tertiary amines are generally considered to be less prone to nitrosamine formation as they require an additional dealkylation step. A review of the published literature combined with recently generated experimental data from nitrosation experiments carried out on several trialkyl amines further informs on the extent that tertiary amines can form N-nitrosamines by reaction with trace levels of nitrite, which may be present during drug substance or drug product manufacture. Simple trialkylamines, amines containing no additional heteroatoms, have been demonstrated to react via a nitrosative dealkylation mechanism that slowly generates a dialkylamine, which in turn nitrosates. This sequence of reactions to generate a N-nitrosamine is approximately 1000-fold slower than the simple nitrosation of a secondary amine of comparable pKa. Therefore, the formation of N-nitrosamines from simple trialkylamines in pharmaceutical products is typically not considered to be a risk. Dialkylanilines are able to access alternative reaction mechanisms and may undergo dealkylative nitrosation with greater ease than simple trialkylamines and therefore require a more focused risk assessment. Finally, certain structurally complex tertiary amines may contain functional groups that can facilitate the formation of N-nitrosamines through resonance and/or inductive electronic effects. Therefore, structures containing highly functionalized tertiary amines require a thorough, compound-specific assessment to determine the level of risk of nitrosamine generation. Note that in situations where higher amounts of nitrosating agents are present, such as when nitrosation chemistry is used during the drug substance manufacturing process, simple trialkylamines should be considered for N-nitrosamine generation during the risk assessment.
中文翻译:

医药产品中叔胺可形成 N-亚硝基二烷基胺程度的考虑
大多数仲胺在亚硝酸盐存在下,在某些条件下,特别是在低pH值下,有可能发生亚硝化,生成N-亚硝胺。通常认为叔胺不易形成亚硝胺,因为它们需要额外的脱烷基化步骤。对已发表文献的综述结合最近对几种三烷基胺进行的亚硝化实验产生的实验数据进一步说明了叔胺可以通过与微量亚硝酸盐反应形成 N-亚硝胺的程度,亚硝酸盐可能存在于原料药或合成过程中。药品制造。简单的三烷基胺(不含额外杂原子的胺)已被证明可以通过亚硝化脱烷基机制进行反应,缓慢生成二烷基胺,进而产生亚硝基化合物。生成N-亚硝胺的这一反应序列比pKa相当的仲胺的简单亚硝化反应慢大约 1000 倍。因此,药品中由简单三烷基胺形成N-亚硝胺通常不被认为是一种风险。二烷基苯胺能够采用替代反应机制,并且比简单的三烷基胺更容易进行脱烷基亚硝化,因此需要更有针对性的风险评估。最后,某些结构复杂的叔胺可含有可通过共振和/或感应电子效应促进N-亚硝胺形成的官能团。因此,含有高度官能化叔胺的结构需要进行彻底的、特定于化合物的评估,以确定亚硝胺生成的风险水平。请注意,在存在较高量亚硝化剂的情况下,例如在原料药生产过程中使用亚硝化化学时,在风险评估过程中应考虑使用简单的三烷基胺来生成 N-亚硝胺。
更新日期:2023-04-19
中文翻译:

医药产品中叔胺可形成 N-亚硝基二烷基胺程度的考虑
大多数仲胺在亚硝酸盐存在下,在某些条件下,特别是在低pH值下,有可能发生亚硝化,生成N-亚硝胺。通常认为叔胺不易形成亚硝胺,因为它们需要额外的脱烷基化步骤。对已发表文献的综述结合最近对几种三烷基胺进行的亚硝化实验产生的实验数据进一步说明了叔胺可以通过与微量亚硝酸盐反应形成 N-亚硝胺的程度,亚硝酸盐可能存在于原料药或合成过程中。药品制造。简单的三烷基胺(不含额外杂原子的胺)已被证明可以通过亚硝化脱烷基机制进行反应,缓慢生成二烷基胺,进而产生亚硝基化合物。生成N-亚硝胺的这一反应序列比pKa相当的仲胺的简单亚硝化反应慢大约 1000 倍。因此,药品中由简单三烷基胺形成N-亚硝胺通常不被认为是一种风险。二烷基苯胺能够采用替代反应机制,并且比简单的三烷基胺更容易进行脱烷基亚硝化,因此需要更有针对性的风险评估。最后,某些结构复杂的叔胺可含有可通过共振和/或感应电子效应促进N-亚硝胺形成的官能团。因此,含有高度官能化叔胺的结构需要进行彻底的、特定于化合物的评估,以确定亚硝胺生成的风险水平。请注意,在存在较高量亚硝化剂的情况下,例如在原料药生产过程中使用亚硝化化学时,在风险评估过程中应考虑使用简单的三烷基胺来生成 N-亚硝胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号