Resources, Conservation and Recycling ( IF 11.2 ) Pub Date : 2023-04-19 , DOI: 10.1016/j.resconrec.2023.107005 Wenhao Yu , Yingchao Zhang , Jiehui Hu , Jiahui Zhou , Zhen Shang , Xia Zhou , Shengming Xu
|
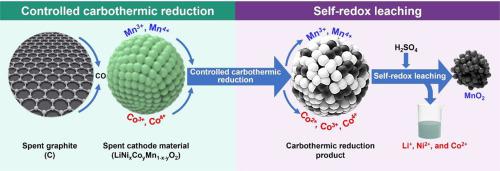
In this study, a controlled carbothermic reduction followed by self-redox leaching process is developed for the stepwise recovery of metals from spent NCM battery. In the controlled carbothermic reduction step, the phase of the product can be precisely regulated simply by controlling the temperature and graphite dosage. In the self-redox leaching step, a redox reaction is carried out between Mn and Co in an H2SO4 solution, resulting in the reduction of Co(III) and Co(IV) to Co2+, and the Mn(II) is oxidized to MnO2 solid product for separation. Under the optimized conditions of H2SO4 concentration of 3 mol/L, leaching temperature of 85 °C, leaching time of 3 h, and liquid-solid ratio of 6 mL/g, the leaching efficiencies of Li, Ni, and Co reached remarkable levels of 99.2%, 98.9%, and 96.4%, respectively, while Mn mostly exists in the form of a solid phase, with a leaching efficiency of only 2.7%.
中文翻译:

可控碳热还原以提高废锂离子电池中金属的回收率
在这项研究中,开发了一种受控碳热还原和自氧化还原浸出工艺,用于从废 NCM 电池中逐步回收金属。在可控碳热还原步骤中,只需控制温度和石墨用量即可精确调节产物相。在自氧化还原浸出步骤中,Mn 和 Co 在 H 2 SO 4溶液中发生氧化还原反应,导致 Co(III) 和 Co(IV) 还原为 Co 2+,而 Mn(II )被氧化成MnO 2固体产物用于分离。在优化的H 2 SO 4条件下在浓度3 mol/L、浸出温度85 °C、浸出时间3 h、液固比6 mL/g的条件下,Li、Ni、Co的浸出率分别达到99.2%、98.9%。 , 和 96.4%,而 Mn 主要以固相形式存在,浸出效率仅为 2.7%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号