Structure ( IF 4.4 ) Pub Date : 2023-04-13 , DOI: 10.1016/j.str.2023.03.013 Jussi Aittoniemi 1 , Morten Ø Jensen 1 , Albert C Pan 1 , David E Shaw 2
|
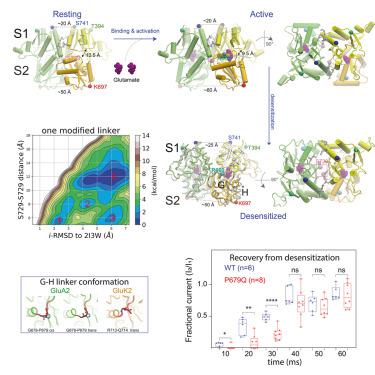
To perform their physiological functions, amino methyl propionic acid receptors (AMPARs) cycle through active, resting, and desensitized states, and dysfunction in AMPAR activity is associated with various neurological disorders. Transitions among AMPAR functional states, however, are largely uncharacterized at atomic resolution and are difficult to examine experimentally. Here, we report long-timescale molecular dynamics simulations of dimerized AMPAR ligand-binding domains (LBDs), whose conformational changes are tightly coupled to changes in AMPAR functional states, in which we observed LBD dimer activation and deactivation upon ligand binding and unbinding at atomic resolution. Importantly, we observed the ligand-bound LBD dimer transition from the active conformation to several other conformations, which may correspond with distinct desensitized conformations. We also identified a linker region whose structural rearrangements heavily affected the transitions to and among these putative desensitized conformations, and confirmed, using electrophysiology experiments, the importance of the linker region in these functional transitions.
中文翻译:

AMPA 受体的脱敏动力学
为了发挥其生理功能,氨基甲基丙酸受体 (AMPAR) 在活跃、静息和脱敏状态之间循环,AMPAR 活性的功能障碍与各种神经系统疾病有关。然而,AMPAR 功能状态之间的转换在原子分辨率下基本上没有特征,并且难以通过实验检查。在这里,我们报告了二聚化 AMPAR 配体结合域 (LBD) 的长期分子动力学模拟,其构象变化与 AMPAR 功能状态的变化紧密相关,其中我们观察到 LBD 二聚体在配体结合和解除结合时的激活和失活解决。重要的是,我们观察到配体结合的 LBD 二聚体从活性构象转变为其他几种构象,这可能与不同的脱敏构象相对应。我们还确定了一个连接子区域,其结构重排严重影响了这些假定的脱敏构象之间的转换,并使用电生理学实验证实了连接子区域在这些功能转换中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号