当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Process Development for the Synthesis of BI 1702135: A Concise Design Enabled by Selective Acylation of a 2-Aminobenzimidazole Intermediate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-04-05 , DOI: 10.1021/acs.oprd.3c00050 Jiang-Ping Wu 1 , Wei-Tong Dong 1 , Chengsheng Chen 1 , Alexander Sienkiewicz 1 , Phouvieng Beyer 1 , Jun Wang 1 , Nizar Haddad 1 , Jon C. Lorenz 1 , Jonathan T. Reeves 1 , Matthew H. Bunner 2 , Nina C. Gonnella 2 , Harald Weinstabl 3 , Géraldine Garavel 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-04-05 , DOI: 10.1021/acs.oprd.3c00050 Jiang-Ping Wu 1 , Wei-Tong Dong 1 , Chengsheng Chen 1 , Alexander Sienkiewicz 1 , Phouvieng Beyer 1 , Jun Wang 1 , Nizar Haddad 1 , Jon C. Lorenz 1 , Jonathan T. Reeves 1 , Matthew H. Bunner 2 , Nina C. Gonnella 2 , Harald Weinstabl 3 , Géraldine Garavel 3
Affiliation
|
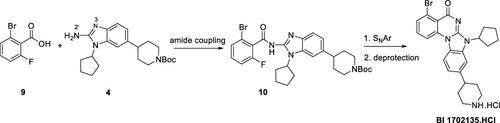
The route design and process development for the synthesis of BI 1702135 are described. The synthetic design centers around an amide synthesis involving a 2-amino benzimidazole analog 4 that has two competing acylation sites. A highly selective acylation protocol at the desired N-2′ site was identified. Robust and convenient processes were developed for each step in this five-step synthesis of BI 1702135.
中文翻译:

BI 1702135 的合成工艺开发:通过 2-氨基苯并咪唑中间体的选择性酰化实现的简洁设计
描述了BI 1702135的合成路线设计和工艺开发。合成设计以酰胺合成为中心,涉及具有两个竞争性酰化位点的2-氨基苯并咪唑类似物4 。确定了所需 N-2' 位点的高选择性酰化方案。为BI 1702135的五步合成中的每一步开发了稳健且方便的流程。
更新日期:2023-04-05
中文翻译:

BI 1702135 的合成工艺开发:通过 2-氨基苯并咪唑中间体的选择性酰化实现的简洁设计
描述了BI 1702135的合成路线设计和工艺开发。合成设计以酰胺合成为中心,涉及具有两个竞争性酰化位点的2-氨基苯并咪唑类似物4 。确定了所需 N-2' 位点的高选择性酰化方案。为BI 1702135的五步合成中的每一步开发了稳健且方便的流程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号