Structure ( IF 4.4 ) Pub Date : 2023-04-04 , DOI: 10.1016/j.str.2023.03.009 Suman Mishra 1 , Anupam Roy 1 , Somnath Dutta 1
|
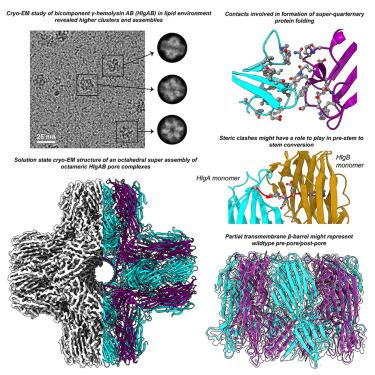
γ-Hemolysin (γ-HL) is a hemolytic and leukotoxic bicomponent β-pore-forming toxin (β-PFT), a potent virulence factor from the Staphylococcus aureus Newman strain. In this study, we performed single-particle cryoelectron microscopy (cryo-EM) of γ-HL in a lipid environment. We observed clustering and square lattice packing of octameric HlgAB pores on the membrane bilayer and an octahedral superassembly of octameric pore complexes that we resolved at resolution of 3.5 Å. Our atomic model further demonstrated the key residues involved in hydrophobic zipping between the rim domains of adjacent octameric complexes, providing additional structural stability in PFTs post oligomerization. We also observed extra densities at the octahedral and octameric interfaces, providing insights into the plausible lipid-binding residues involved for HlgA and HlgB components. Furthermore, the hitherto elusive N-terminal region of HlgA was also resolved in our cryo-EM map, and an overall mechanism of pore formation for bicomponent β-PFTs is proposed.
中文翻译:

基于冷冻电镜的对金黄色葡萄球菌 γ-溶血素超分子组装体的结构洞察揭示了孔隙形成机制
γ-溶血素 (γ-HL) 是一种溶血性和白细胞毒性双组分 β-成孔毒素 (β-PFT),是金黄色葡萄球菌Newman 菌株的一种强毒力因子。在这项研究中,我们在脂质环境中对 γ-HL 进行了单粒子冷冻电镜 (cryo-EM)。我们观察到膜双层上八聚体 HlgAB 孔的聚类和方形晶格堆积,以及我们以 3.5 Å 分辨率解析的八聚体孔复合物的八面体超组装。我们的原子模型进一步证明了参与相邻八聚体复合物边缘结构域之间疏水性压缩的关键残基,为 PFT 后的结构提供了额外的结构稳定性寡聚化。我们还在八面体和八聚体界面处观察到额外的密度,从而提供了对 HlgA 和 HlgB 成分所涉及的可能的脂质结合残基的见解。此外,HlgA 迄今难以捉摸的 N 末端区域也在我们的冷冻电镜图中得到解决,并提出了双组分 β-PFT 孔形成的总体机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号