Structure ( IF 4.4 ) Pub Date : 2023-03-30 , DOI: 10.1016/j.str.2023.03.005 Matthew H Doran 1 , Joseph L Baker 2 , Tobias Dahlberg 3 , Magnus Andersson 4 , Esther Bullitt 1
|
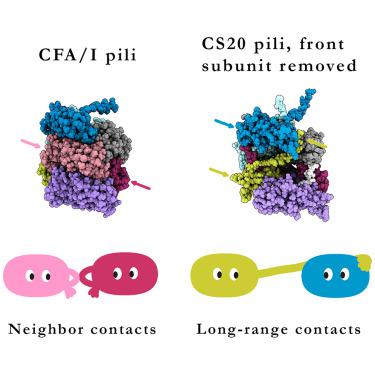
Bacterial adhesion pili are key virulence factors that mediate host-pathogen interactions in diverse epithelial environments. Deploying a multimodal approach, we probed the structural basis underpinning the biophysical properties of pili originating from enterotoxigenic (ETEC) and uropathogenic bacteria. Using cryo-electron microscopy we solved the structures of three vaccine target pili from ETEC bacteria, CFA/I, CS17, and CS20. Pairing these and previous pilus structures with force spectroscopy and steered molecular dynamics simulations, we find a strong correlation between subunit-subunit interaction energies and the force required for pilus unwinding, irrespective of genetic similarity. Pili integrate three structural solutions for stabilizing their assemblies: layer-to-layer interactions, N-terminal interactions to distant subunits, and extended loop interactions from adjacent subunits. Tuning of these structural solutions alters the biophysical properties of pili and promotes the superelastic behavior that is essential for sustained bacterial attachment.
中文翻译:

细菌粘附菌毛稳定性和超弹性的三种结构解决方案
细菌粘附菌毛是介导不同上皮环境中宿主-病原体相互作用的关键毒力因子。我们采用多模式方法,探讨了支撑产肠毒素 (ETEC) 和尿路致病菌菌毛生物物理特性的结构基础。我们使用冷冻电子显微镜解析了来自 ETEC 细菌、CFA/I、CS17 和 CS20 的三种疫苗靶菌毛的结构。将这些和以前的菌毛结构与力谱和操纵分子动力学模拟配对,我们发现亚基-亚基相互作用能与菌毛展开所需的力之间存在很强的相关性,而与遗传相似性无关。Pili 集成了三种结构解决方案来稳定其组件:层间相互作用、N 端与远距离亚基的相互作用、和来自相邻亚基的扩展环相互作用。这些结构解决方案的调整会改变菌毛的生物物理特性,并促进对持续细菌附着至关重要的超弹性行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号